The Role of Dexamethasone in the Era of “Dex-CSDH” Randomized Controlled Trial. A Multicenter, Prospective Study on Specific Subset of Patients with Chronic Subdural Hematoma (CSDH) Treated with Dexamethasone Alone or Surgery
Journal of Head Neck & Spine Surgery Juniper Publishers

Abstract
Background: The efficacy of Dexamethasone as a method of conservative management for CSDH to avoid surgery remains inconclusive even after “Dex-CSDH” randomized controlled trial as almost all patients underwent an initial operation to remove the hematoma. Current study aims to determine the efficacy and safety of Dexamethasone alone therapy in comparison with the surgical drain of CSDH.
Methods: A multicenter, prospective study on mild to moderately symptomatic adult patients with CSDH were performed from January 2019 to December 2020. The patients were enrolled in a 1:1 ratio to receive a 3-weeks tapering course of oral Dexamethasone, starting at 4 mg thrice daily, or surgical drain of CSDH. The patient was assigned to a particular therapy based on their preference. The primary outcome was functional outcome in each group at 6 months determined by mRS, and cure rate (symptomatic relief and radiological resolution of CSDH).
Results: Twenty eights patients were assigned to Dexamethasone group and 30 patients to surgical group. The mean age of the patient was about 62 years in both groups with male predominance (>60%). A favorable outcome determined by mRS scale of 1 to 3 at 6 months follow-up was 25/28 (89.2%) in Dexamethasone group compared to 28/30 (93.3%) in surgical group (difference, -4.1 [95% CI, -6.8 to -2.2], p=0.12). The cure rate was 23/28 (82.1%) in Dexamethasone group compared to 28/30 (93.3%) in the surgical group during index admission (difference, -11.2, [95% CI, -14.3 to -7.6] in favor of the surgical group, p=0.005). Recurrence rate for CSDH was 2/28 (7.1%) in Dexamethasone group compared to surgical group 4/30 (13.3%) which was statistically significant (p=0.01). Morbidity related to medical therapy was not statistically higher compared to surgical group.
Conclusion: Current study exhibits superiority of surgery over Dexamethasone therapy for resolution of symptomatic CSDH. The recurrence rate of CSDH is lower in patients with corticotherapy compared to surgical drain. Patients in Dexamethasone group did not have functional outcome at 6 months or morbidity significantly higher than surgical group. This study strongly puts forward the utility of Dexamethasone as an effective and safe alternative to surgery for CSDH.
Keywords: Chronic subdural hematoma; Dexamethasone; Surgery; Functional outcome; Corticotherapy
Abbreviations: CSDH: Chronic Subdural Hematoma; CT: Computed Tomography; GCS: Glasgow Coma Scale; MRS: Modified Rankin Score; FFP: Fresh Frozen Plasm
Introduction
Chronic subdural hematoma (CSDH) is a common neurological disease prevalent in elderly population and has shown an increase in incidence due to extended life expectancy and use of blood thinners [1]. Burr-hole evacuation of CSDH is simple and easy to perform procedure with low morbidity rate (ranging from 0 to 9%) [2], but a rather variable rate of recurrence (10 to 20%) [3,4]. Surgery remains the mainstay therapy for patients with symptomatic CSDH with acceptable morbidity in medical community which produces excellent outcome and good symptomatic relief. Despite the excellent outcomes obtained by surgery, complications may occur, some of which may be potentially severe or fatal. Furthermore, up to 25% recurrence rate is reported.
Glucocorticoid has been used to treat CSDH [5], with acceptable efficacy and safety profile as stand-alone therapy to avoid surgery or as an adjunct to surgery to prevent recurrence of subdural fluid collection [6,7]. The Dexamethasone for Adult Patients with Symptomatic Chronic Subdural hematoma (Dex-CSDH) trial was a multicenter, randomized control trial conducted in the United Kingdom and published in December 2020 [8]. This recently concluded study aimed to determine the effect of Dexamethasone on outcomes in patients with symptomatic CSDH. It reported that most of patients (>90%) had to undergo surgical drainage of CSDH while on 2 weeks tapering dosage of Dexamethasone drug, which resulted in fewer favorable outcome and more adverse events compared to placebo at 6 months, but fewer recurrence of subdural fluid collection in the Dexamethasone group.
This provided much needed Class I evidence on Dexamethasone therapy to evaluate its effectiveness and its impact on the functional outcome of the affected patients. Since almost all the patients underwent surgery to evacuate CSDH while of Corticotherapy, the true effect of Dexamethasone as stand-alone therapy to treat this disease effectively, safely, with avoidance of surgery and prevent recurrence of subdural collection could not be ascertained by this study. Current study is a multicenter, nonrandomized but controlled, prospective clinical trial to determine the efficacy, safety and functional outcome of patients with standalone 3 weeks tapering dose of Dexamethasone medical therapy compared to surgical drainage of CSDH. Our study aims to add further clarity to the role of corticotherapy in conjunction with Dex-CSDH study in patients with chronic subdural hematoma.
Materials and Methods
Study design
This study is a multicenter, prospective clinical trial that was conducted in 4 different hospitals in Kathmandu, Nepal where the author treated the patients with CSDH. Patients were enrolled in stand-alone 3 weeks tapering course of Dexamethasone or burrhole evacuation of hematoma as the treatment modality based on patient’s preference. To be precise, the decision to perform surgical evacuation of subdural hematoma or conservative monitoring was made by the author following detailed discussion with the patient and patient’s next of kin. Hence it is a non-randomized study but a controlled study. The duration of the study was from January 2019 to December 2020 (2 years). We had almost 1:1 ratio between the two cohorts of patients. Dexamethasone group had total of 28 patients, while there were 30 patients in the surgical group who met eligibility criteria and fulfilled follow-up requisite.
Informed written consent was obtained for all patients who had surgery for CSDH. Verbal consent was obtained and noted in the medical records who preferred stand-alone Dexamethasone therapy as the treatment modality for CSDH. A chronic subdural hematoma was defined as a predominantly hypodense or combination of hypodense with isodense (relative the brain grey matter) crescentic collection along the cerebral convexity on computed tomography (CT) of the head.
Eligibility criteria
Inclusion Criteria: 1) The hematoma has be to be chronic or predominantly chronic with component of subacute blood 2) Glasgow Coma scale (GCS) on initiation of both alternative therapies of 13-15, 3) Modified Rankin Score (mRS) of 1-3 (see below for details on mRS scoring system) 3) Strict adherence to 3 weeks regimen of Dexamethasone as per the prescribed format [see below] 4) Follow-up on a weekly basis for patients on corticosteroid therapy to look for complications or adverse events associated with it 4) Follow-up CT scan every week for patients on corticosteroid therapy and before hospital discharge for index admission of patients with surgical evacuation of hematoma 5) Trauma or spontaneous CSDH were included in the study 6) Patients on anticoagulant or antiplatelet agent when CSDH was discovered 6) Patients who were available for evaluation of their clinical status, functional outcome and repeat CT head at 3 and 6 months follow-up visit after completion of their respective therapies.
Exclusion Criteria: 1) Subdural hygroma 2) Patients on antiplatelet or anticoagulants 3) Asymptomatic patients 4) Patients with poor neurological or functional status as is evident in inclusion criteria, GCS <13 and mRS>3 were excluded as per the pre-fixed study design [explained in discussion part] 4) Contraindications for glucocorticoid use (e.g. uncontrolled diabetes mellitus, recent peptic ulceration or gastrointestinal bleeding, severe valvular heart disease or unstable angina pectoris, active systemic infection, lactose intolerance, had a cerebrospinal fluid diversion shunt, known hypersensitivity to Dexamethasone, had a history of psychotic disorder and patients receiving glucocorticoid oral or intravenous within the last 1 month 5) Patients who failed Dexamethasone therapy and had subsequent surgical evacuation of CSDH were not included in the surgical arm of patients 7) any pre-existing medical condition that precludes surgical drainage of CSDH.
Patients
Patients with symptomatic CSDH who were eligible for the study were explained the pros and cons about both forms of treatment modalities. They were explicitly told that Dexamethasone as a stand-alone treatment may not lead to resolution or significant reduction of CSDH, which may ultimately lead to surgical drainage of blood to alleviate the neurological symptoms. This drug has inherited complications/side effects which needs to be carefully monitored during the prescribed period of treatment. The pros and cons of surgical drainage of subdural fluid collection through a burr-hole was also explained along with the complications related to surgical procedure.
Treatment protocol
All patients in this study were enrolled with intention to treat mindset using two different treatment protocol. All the patients under the surgical arm underwent standard single or double burr-hole evacuation of CSDH (based on surgeon’s discretion) with placement of subdural drain to a closed system for 1-2 days in the hospitals with neurosurgical units. The anticoagulant and antiplatelet effect were reversed with fresh frozen plasm (FFP) and platelet infusion if there is urgency to perform surgical drain of CSDH. Otherwise, about 10 days was awaited after discontinuation of blood thinners before performing surgery. Patients who had steroid therapy received a tapering 3-weeks course of oral Dexamethasone at home or assisted living facility (4mg thrice daily on days 1 to 3, then 3mg thrice daily on days 4 to 6, then 2mg thrice daily on days 7 to 9, then 2mg twice daily on days 10 to 12, then 1mg thrice daily on days 13 to 15, then 1mg twice daily on days 16-18, then 1mg once daily form 19-21 days and then stopping the therapy). A proton pump inhibitor was given during corticotherapy to prevent steroid induced peptic ulceration.
Patients who had dexamethasone therapy for resolution of CSDH were evaluated on outpatient basis weekly with fasting and post-prandial blood sugar and blood tests for the presence of newonset diabetes and hypokalemia respectively, steroid induced new-onset psychosis, peptic ulceration and gastrointestinal bleeding, and other gastrointestinal side effects. Adherence to medical therapy was confirmed by reviewing patient’s or next of kin medication records and patient diaries to confirm that the medication was taken at home. Patients who were symptomatic and CT head showed mass effect from persisting CSDH despite completion of 3 weeks of Dexamethasone therapy were given option to undergo surgery to evacuate the hematoma. If agreed upon, these select group of patients underwent standard burrhole evacuation of CSDH with subdural drain.
Outcomes
The author believes that Dexamethasone is a potent antineomembrane and anti-angiogenic agent which is safe and effective treatment for CSDH that positively compares to surgical drain of the subdural collection. The author does not intend to undermine the importance of surgical evacuation of CSDH, but Dexamethasone can be a viable option, especially in specific circumstances, especially in patients with CSDH with minimal or moderate symptomatology and not severely affected neurologically or functionally. In these select subset of patients with chronic subdural hematoma, corticotherpay can be a conservative alternative treatment option in comparison to surgical drain of subdural blood collection.
Ethical issues seems to be a non-entity in this study considering patient or his/her next of kin chose the treatment modality on their own accord which has proven to be effective backed by evidence based medicine and substantial literature on both modalities of treatment for CSDH [8,9]. The modified Ranking scale (mRS) is an ordinal scale to evaluate patient’s functional status with respect to activities of daily living and has wide spread clinical application for several diseases [10,11]. This scale has seven categories: no symptoms-0, no clinically significant disability despite symptoms-1, slight disability-2, moderate disability-3, moderately sever disability-4, severe disability-5, and death-6; in this study, a score of 0 to 3 was used to indicate a favorable outcome.
In lieu with the previous studies of CSDH [12,13], the primary outcome was 1) a score of 0 to 3 on the mRS at 6 months after enrollment in the study, suggesting favorable outcome 2) resolution or significant reduction of chronic subdural collection at the end of index admission for the surgical group and at the end of 3 weeks of dexamethasone therapy for medical group (designated Cure Rate in current study). Resolution of SDH was defined as above 90% reduction in the thickness of the fluid collection with symptomatic relief. Significant reduction was described as above 75% reduction in the thickness of the subdural collection of blood with symptomatic relief.
Secondary outcome was the score on mRS at the end of each respective therapies and at 3 months interval after completion of respective treatment modalities, the number of patients who had to undergo surgical interventions during subsequent admission in the follow-up period. This entails surgery for recurrence of subdural hematoma following burr-hole evacuation of subdural collection in the surgical group or after corticotherapy. Surgical intervention in patients who had corticotherapy and could not effect a cure is also reported in current study. Post-operative recurrence of CSDH was defined as symptomatic re-accumulation of the ipsilateral subdural fluid that was previously evacuated.
Tertiary outcome was mortality at 30 days and 6 months after enrollment of patients in two distinct therapeutic modalities. It also includes morbidity related to both medical and surgical therapies.
Statistical Analysis
The two cohort of patients (surgical group vs Dexamethasone group) were matched for age, sex, GCS on presentation and initial modified Rankin Score. Overall effect was shown with the risk ratio (RR) and its 95% confidence interval (95% CI). Comparisons between Dexamethasone alone therapy versus the surgical drain were made to identify the effectiveness of Dexamethasone therapy. A two-tailed P value < 0.05 set as the level of significance under the assumption of a normal distribution curve in the current study. Statistical significance between two groups was examined by Pearson Chi-Square test or Fisher exact test for categorical variables and Student t-test or Mann-Whitney U-test for continuous variables respectively. Multivariate Logistic Regression analysis models (using the ordinal modified Rankin Scale) [14] were performed to compare variables between two select cohorts.
Results
The age in each group was about 62 years, with male predominance (64% in Dexamethasone group and 67% in the surgical group) (Table 1). The most common cause of CSDH, irrespective to the assigned group for patients with CSDH was head trauma (about 67%), followed by use of blood thinners and spontaneous in nature (14.2% and 18% in Dexamethasone group, and 16.7% and 16.7% in the surgical group respectively). Head, gait instability and cognitive impairment were the most common presenting symptoms in descending order (71.4%, 53.6% and 43% in Dexamethasone group as compared to surgical group (70%, 56.6% and 46.6% respectively). Ischemic heart disease, diabetes mellitus and chronic obstructive pulmonary disease were the most common presenting morbidity in each group (43%, 28.5% and 21.4% in Dexamethasone group compared to 46.6%, 33.3% and 26.6% respectively). The midline shift in brain due to mass effect from ipsilateral CSDH is most commonly in the range of 6-10 mm as evident on CT head (43% and 46.6% in Dexamethasone group and surgical group respectively). The patients who underwent surgery during index admission for surgical drain of SDH mean stay with SD was 5 days ±1.8.
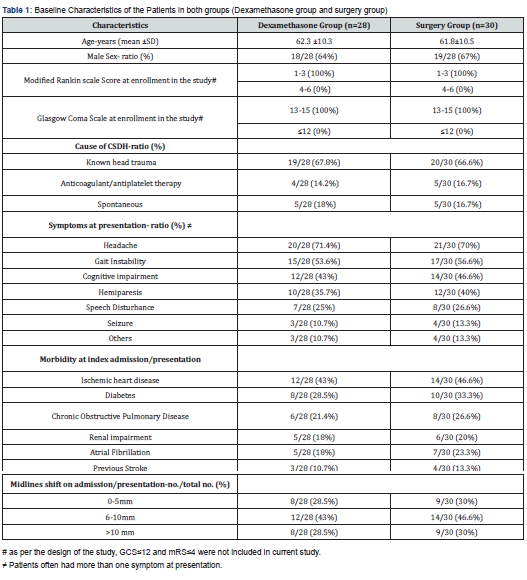
Primary outcome
A favorable functional outcome determined by modified Rankin scale (mRS) score of 1 to 3 at 6 months follow-up was 25/28 (89.2%) in Dexamethasone group compared to 28/30 (93.3%) in surgical group (difference, -4.1 [95% CI, -6.8 to -2.2], p value =0.12). The difference was not statistically significant. The cure rate was 23/28 (82.1%) in Dexamethasone group compared to 28/30 (93.3%) in the surgical group during index admission (difference, -11.2, [95% 95% CI -14.3 to -7.6] in favor of the surgical group, p=0.005). About 18% patients (5/28) had to undergo surgical drain of subdural collection for failure to resolve following full course of corticotherapy.
Secondary outcome
A favorable functional outcome (mRS score of 1-3) at 3 months follow-up was 26/28 (92.8%) in Dexamethasone group compared to 28/30 (93.3%) in surgical group (difference, -0.5 [95% CI, -2.2 to -0.1], p value =1.24 (Table 2). It was comparable between 2 groups at 3 months follow-up. Similarly, at the time of discharge for surgical group during index admission and end of therapy for oral dexamethasone group, favorable outcome determined by mRS was not statistically significant. It was 25/28 (89.2%) in Dexamethasone group compared to 28/30(93.3%) [Point Difference -4.1 (-8.8 to -2.2), p value= 0.08]. Hence at no point in time, surgical group had significantly better outcome compared to corticotherapy group. Only 3 patients (10%) in the surgical group had to undergo repeat evacuation of subdural fluid collection during index admission after first surgery failed to resolve CSDH. Three patients (10%) in the surgical group had subsequent surgery for tension pneumocephalus, contralateral acute subdural hematoma and acute intracerebral hemorrhage (the latter probably due to brain laceration from the subdural irrigation catheter). Recurrence rate for CSDH was 2/28 (7.1%) in Dexamethasone group compared to surgical group 4/30 (13.3%) [Point difference of –6.2 (-4.2 to -8.4)] which was statistically significant (p=0.01).
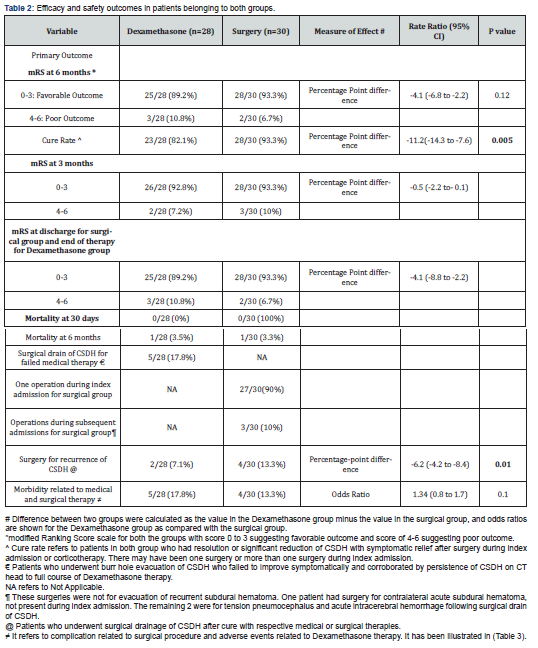
Tertiary outcome
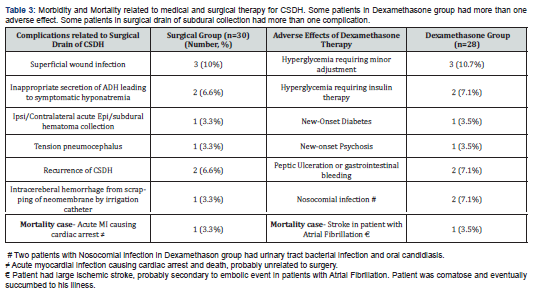
Morbidity related to stand alone Dexamethasone therapy and surgical drainage of CSDH were 17.8% (5/28 patients) compared to 13.3% (4/30) with Rate Ratio of 1.34 (range 0.8 to 1.7) with p value of 0.10. Hence morbidity related to corticotherapy did not reach statistical significance compared to the surgical therapy. The most common adverse effects related to medical therapy (Table 3) was hyperglycemia 17.8% (5/28 patients), followed by gastrointestinal problems including peptic ulceration and nosocomial infection, latter two representing 2/28 patients each (7.1%). In the surgical group, the most common complications were superficial wound infections in 3 out 30 patients (10%) and inappropriate secretions of ADH leading to hyponatremia (2/30 patients, 6.6%). They were managed with appropriate antibiotics and water/fluid restriction. No morbidity related to either therapy were life threatening in this study. There was no mortality at the time of discharge after surgery or end of medical therapy, same applies at 3 months follow-up period. Mortality was 1 patient each in assigned groups at 6 months follow-up, which was not directly related to treatment instituted.
Discussion
Chronic subdural hematoma is a common but retractable neurological disease in the elderly with a noteworthy recurrence rate (up to 25% has been reported in the literature) [9]. The pathophysiology of CSDH has been well elucidated. The process of re-bleeding and breakdown of erythrocytes exacerbated the inflammatory reaction, following by the formation of neomembrane and neo-capillaries. This cycle of the re-bleedingcoagulation- fibrinolysis process eventually led the hematoma to enlarge or recur [9]. Glucocorticoid, a potent anti-inflammatory agent has been found to inhibit neo-membrane and neoangiogenesis [15]. This has been the rationale for the use of Dexamethasone orally or intravenously to treat CSDH.
A review and meta-analysis study on Dexamethasone role for CSDH concluded even though corticotherapy has been used as stand-alone medical therapy, it’s effectiveness as a better alternative to surgical therapy has not been proven [9]. But adjuvant Dexamethasone therapy to surgical drain of CSDH is known to reduce the recurrence of this disease. Trial of Dexamethasone for Chronic Subdural Hematoma (Dex-CSDH) provided much needed multicenter randomized controlled study to elucidate efficacy and safety of Dexamethasone compared to placebo in treating symptomatic CSDH [8]. In this study, almost all patients underwent an initial operation to remove hematoma, no firm conclusion could be drawn regarding the effect of Dexamethasone as a method of conservative management to avoid surgery. However, it did further strengthen the existing literature about the potency of corticotherapy to avoid recurrence of chronic subdural hematoma.
Current study is inspired by anecdotal experience of the author in successfully implementing corticotherapy as standalone therapy for symptomatic CSDH. In his experience, he got better results from 3 weeks of tapering regimen of oral Dexamethasone drug as compared to routinely used 2 weeks of corticotherapy in respect to resolution of subdural fluid collection. This was achieved with acceptable adverse effects related to considerable period of glucocorticoid therapy with strict vigilance of patient during this therapy. When pilot study on Dex-CSDH was published in 2019 with its inception in 2015 [16], the author was enthusiastically propelled to contemplate similar study in the country of his medical practice. The author wanted to conduct a randomized controlled trial comparing the efficacy and safety of Dexamethasone therapy with surgical drain of subdural collection.
Since patient were not keen on randomization and their preference of therapy was paramount, it ended up being a multicenter, prospective, non-randomized but controlled study. The author believed that CSDH patients with poor neurological and functional status should be subjected to surgical drainage for urgent alleviation of their symptomatology and neurological recovery. Hence, mild to moderately symptomatic patients with favorable functional outcome were only enrolled for corticotherapy (GCS of 13-15 and mRS score of 1-3). Being a comparative and controlled study, the surgical group also constituted patients with similar neurological and functional status as Dexamethasone group. All the eligible patients who were assigned to either Dexamethasone group or surgical group were matched for age, sex, GCS score and mRS score at the time of enrollment in the study.
Current study shows that at 6 months follow-up, functional outcome of patients based on modified Rankin Score scale was not statistically significant between stand-alone Dexamethasone group and surgery group. Similar picture emerged for patient’s functional outcome at end of respective therapies or at 3 months following discharge from medical care. However, the cure rate which specified significant or complete resolution of CSDH with symptomatic relief was statistically significantly higher in surgical arm compared to medical therapy (82.1% for Dexamethasone group vs 93.3% for surgery group, P value of 0.005). The recurrence rate for CSDH in Dexamethasone group was lower compared to surgical group which reached statistical significance (7.1% for corticotherapy vs 13.3% for surgical group, P value of 0.01). This is line with most of the studies in existing literature and corroborated by Dex-CSDH study [8].
As shown by various study including Dex-CSDH trial , in this study adverse effects or morbidity related to Dexamethasone therapy was not markedly higher compared to surgical therapy (not statistically significant on analysis). None of the patients in this study had life threatening complications related to respective therapies. About 18% (5 out of 28 patients) in medical treatment group had failure of therapy to resolve CSDH, hence underwent surgical evacuation of the subdural collection. This is in contrast to 2 patients (6.7%) who failed to be cured by surgical evacuation of subdural hematoma at the end of index admission. These two patients had multi-loculated subdural hematoma which prevented from surgical cure. In totality, 3 patients had re-do surgery for removal of residual and symptomatic CSDH during index admission through burr-hole drainage. Three patients (10%) in the surgical group had subsequent surgery unrelated to recurrence of CSDH (e.g., tension pneumocephalus, contralateral acute subdural hematoma collection etc.). There was one mortality among patients belonging to both types of therapies at 6 months follow-up, but probably unrelated to the instituted therapies.
Our trial has limitations, the most important being a nonrandomized trial and small sample size. Another arm of patients with Dexamethasone as an adjunctive treatment to surgery would have added more clarity and substance to this study.
Conclusion
Current study exhibits superiority of surgery over Dexamethasone therapy for resolution of symptomatic chronic subdural hematoma. The recurrence rate of CSDH is lower in patients with corticotherapy compared to surgical drain. Patients in Dexamethasone therapy did not have morbidity significantly higher than surgical group. This study strongly puts forward the utility of Dexamethasone as a viable alternative to surgery for CSDH. It can be a safe and effective stand-alone therapy especially in subset of CSDH patients with initial good neurological and functional status.
To Know More About Journal of Head Neck & Spine Surgery Please click on:
For more Open Access Journals in Juniper Publishers please click on:
Comments
Post a Comment