Expect the Unexpected with Erector Spinae Plane Block in Spine Surgery - Plan for the Worst and Hope for the Best: An Anesthesiologist Perspective
Journal of Head Neck & Spine Surgery Juniper Publishers
Abstract
Spine surgery is associated with multiple postoperative complications, ranging from simple nausea and vomiting to devastating complications leading to postoperative morbidity or mortality. The postoperative neurological impairment, especially in the neurologically intact patient, is a dreadful event that makes it difficult for the surgeon to perform technically challenging or high-risk spine surgeries. Preoperative or intraoperative factors that can influence the postoperative neurological status include nature and the severity of the pathology, comorbid conditions of the patient, preexisting neurological symptoms, multiple levels involved, complex surgery or instrumentation, surgical blood loss, neurological monitoring, hemodynamic parameters, polypharmacy, and total duration of the surgery.
In addition to several known contributing factors (fixation failure, epidural hematoma, spinal cord edema, and ischemia-reperfusion injury), the role of the erector spinae plane block (ESPB) has recently been cited as a potential cause of postoperative transient paralysis after spine surgery. ESPB is considered a simple and safe regional anesthesia technique that may have an advantage in success rate and analgesic efficacy when used as an adjunct to general anesthesia in spine surgeries. Despite varied patterns of the drug spread, ESPB has been showing promising results due to consistent involvement of dorsal rami that supply all pain generators of the spine surgeries.
The potential role of ESPB in causing postoperative transient neurological complications is a diagnosis of exclusion that requires thorough clinical assessment and step-by-step evaluation using imaging modalities. Before administering ESPB in spine surgery, essential knowledge includes anatomical and technical considerations, drug distribution patterns, safe and effective volumes/types of local anesthetics, and possible associated complications. This review article describes the possible roles of all factors that lead to postoperative neurological impairment and suggests some tips and tricks for using ESPB in spine surgeries to prevent or manage such serious complications appropriately.
Keywords: Transient paraplegia; Erector spinae plane block; ESP block complications; ESP block in spine surgery; Paraplegia due to RA
Abbreviations: RA: Regional anesthesia; GA: General anesthesia; ESPB: Erector spinae plane block; ERAS: Enhanced recovery after surgery; LA: Local anesthetics; CT: Computed tomography; MRI: Magnetic resonance imaging; ESM: Erector spinae muscles; TP: Transverse process; SMPB: Sacral multifidus plane block; RLB: Retrolaminar block
Introduction
The occurrence of perioperative complications may be inevitable, but their prevention and management are always a shared responsibility of all team members involved. Thorough evaluation of such complications will help develop strategies to prevent and manage the same in the future. A systematic and stepwise approach is warranted before categorizing it as a surgical or anesthetic complication. Several interventions have been introduced in the surgical and anesthetic techniques to improve patient safety and satisfaction. Application of regional anesthesia (RA) alone or as an adjunct to general anesthesia (GA) is one such advance that helps reduce many polypharmacy-related side effects or complications. If a particular complication-reduction modality is inherently causing complications, it requires a comprehensive understanding of the situation and its contributing factors.
An erector spinae plane block (ESPB), a safe and simple RA technique, has shown promising results as an adjunct to multimodal analgesia in various orthopedic, general, thoracic, abdominal, obstetrics, and spine surgeries. In addition to its superior postoperative analgesic profile in spine surgeries at various levels, ESPB reduces hospitalization costs and the possible side effects of extensive anesthetic use. Since opioids have been linked to tumor recurrence [1,2], ESPB also reduces the risk of spine tumor recurrences by significantly reducing its consumption. ESPB meets all criteria suitable for enhanced recovery after surgery (ERAS) protocol [3] by facilitating early discharge and mobilization of patients. Being a novel RA technique, not many complications have been reported so far except for some anecdotal reports of bilateral quadriceps weakness, transient apathy or aphasia, minor neurological complications due to inadvertent intravascular injection of local anesthetics (LA) [4].
Recently, it has been described as a potential cause of transient paralysis after spine surgeries [5]. Therefore, it is essential to understand the differential diagnoses of postoperative neurological impairment, follow the step-by-step approach to rule them out one by one, determine the possible role of ESPB in their development, and learn the tricks for safely administering ESPB during spine surgery. This review article elaborates the essential background knowledge required before and after the administration of ESPB in spine surgeries.
Discussion
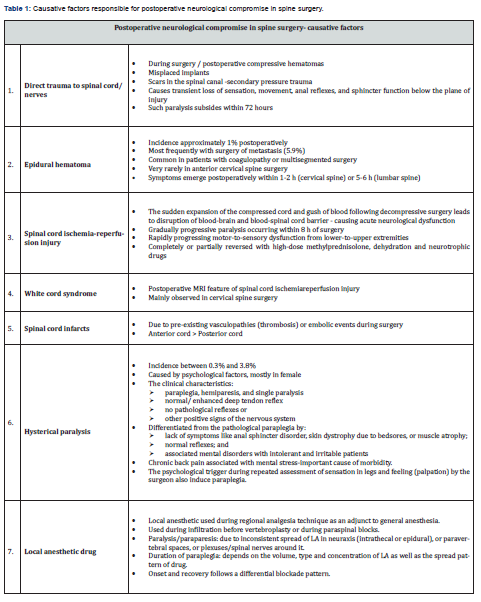
Postoperative neurological impairment after spine surgery in a neurologically intact patient is always daunting for the operating surgeon and the patient. Several common theories on neurological deterioration after decompressive spine surgeries include vascular compromise, hypotension, ischemia, direct trauma, or stretching of the neural elements. The major contributing factors of acute paralysis following spine surgery include fixation failure, epidural hematoma, spinal cord edema, and ischemia‑reperfusion injury [6].
Contributory factors
Neurons in the spinal cord are susceptible to ischemia and hypoxia. The mechanisms of spinal cord ischemia are multi-factorial and multi-channel. The pathogenesis of spinal cord lesions after spine surgeries is usually mechanical (pressure) damage via extensive hematoma or edema, resulting in pressure on the spinal cord leading to ischemic damage [7]. An altered cerebrospinal fluid flow dynamic may also cause cord compression [8]. In either case, the ultimate pathogenic cause is a secondary cellular injury due to the disruption of ionic homeostasis, development of free radicals, lipid oxidation, and degeneration of the cytoskeleton [7]. White cord syndrome, an imaging feature of spinal cord ischemia [9], is diagnosed as high intramedullary signal changes on sagittal T2 weighted MRI scans and is often seen in surgeries on the cervical spine.
The spinal infarct is one of the leading causes of paraplegia or quadriplegia in patients with preexisting vascular pathologies (thrombosis) or embolic events during surgery [10]. The anterior spinal cord has a higher risk of ischemia due to fewer anterior spinal artery feeding vessels [10] than the highly vascular posterior spinal cord due to anastomotic pial vessels. The sparing of the posterior column leads to unchanged intraoperative somatosensory evoked potentials [11]. The ischemia-reperfusion injury occurs upon restoring the blood flow to previously ischemic tissues and organs. Increased inflammatory cytokines such as TNF α and IL 1β may be considered vital indicators for evaluating decompression-associated spinal cord ischemia-reperfusion injury [12,13]. Its reported incidence is 2-5.7% following cervical and 14.5% following posterior thoracic decompression surgeries [14, 15].
Transient paralysis is one such complication that manifests itself as a temporary (up to 72 hours) loss of sensations, movements, anal reflexes, and sphincter function below the affected spinal segments [16]. It can occur after vertebroplasty, laminectomy, or thoracic decompressive procedures [17,18]. The longer duration of symptoms, multiple compression sites, and the high degree of preoperative stenosis are considered poor prognostic factors [18].
Who is the culprit?
The exact cause of the postsurgical neurological impairment is a diagnosis of exclusion requiring thorough clinical evaluation and imaging guidance to rule out each contributing factor (Table 1) in a step-by-step manner. Postoperative radiographic studies like computed tomography (CT) scan and magnetic resonance imaging (MRI) can help detect changes suggestive of misplaced implants, hematomas, edema, compressive lesions, white cord syndrome, or direct trauma to the spinal cord. Symptoms due to spinal cord edema typically occur at 48-72 hours post-surgery and may be relieved by anti-edema measures like fluid restriction [19].
The occurrence and severity of ischemia-reperfusion injury correlate with tissue ischemia time, the extent of ischemic tissue, and the oxygen requirement of the affected tissue [20]. The presence of deep tendon and superficial reflexes may rule out the possibility of hysterical paraplegia [18]. After excluding all contributing factors that may cause postoperative neurological impairment, the possible role of ESPB and LA can be considered and further evaluated. It requires an understanding of the anatomical and technical aspects, mechanism of drug spread, factors favoring neuraxial spread, and measures to avoid such incidents in the future [21].
Role of ESPB
ESPB involves depositing the local anesthetic solution between the erector spinae muscles (ESM) and the transverse process (TP) under ultrasound guidance. The ESM consists of three muscles: iliocostalis, longissimus, and spinalis. They arise from and insert into various bony components of the vertebral column [22] and form a paraspinal column that extends from the sacrum to the base of the skull. It gradually tapers upwards in the paravertebral groove on either side of the spinous processes. The retinaculum (thoracolumbar fascia in the lumbar region) that envelops this muscular column also facilitates the LA spread to several thoracic and lumbosacral levels [23]. The diverse multilayered fascial arrangement deep to the ESM may cause the inconsistent LA spread, resulting in multisegmented sensory block mainly involving dorsal rami with sometimes ventral rami.
This Para neuraxial block, when given bilaterally in spine surgery, can be advantageous in success rate and analgesic efficacy [24]. The absence of risks such as hypotension, vascular spread, or pneumothorax makes ESPB relatively safer than epidural anesthesia or paravertebral block. Bilateral ESPB offers effective perioperative analgesia without influencing the hemodynamic parameters. It significantly reduces the perioperative opioid requirements in spine surgeries at various levels (cervical, thoracic, and lumbar, and sacral) [25-32]. Its outcome depends on the volume and concentration of LA used, drug spread, and the anesthesiologist’s experience in selecting and locating the correct level of the TP.
The exact mechanism of action of the ESP block and pattern of the drug spread is still unclear. It has been suggested to anesthetize the spinal nerves by passing through the costotransverse foramen of Cruveilhier, accompanying the dorsal ramus and artery to the paravertebral space [33]. The deposited drug can spread in any direction, such as craniocaudal, anterior-posterior, and lateral-medial planes to reach the paravertebral space, neural foramina, epidural space, or sympathetic chain [34-38]. Fluoroscopic, CT, and MR imaging in living subjects have similarly confirmed the injectate tracking to the paravertebral area, intervertebral foramina, and epidural space following lumbar ESPB [39-42]. There is also a possibility of LA diffusion through the microscopic gaps in the mostly acellular architecture of interlinked collagen fibers of the fascia covering the erector spinae muscle [43].
ESPB at various spine levels
The anatomical differences at the various spine levels can cause varied drug spread and ultimately affect the outcomes of ESPB. Cervical ESPB is technically challenging due to the difficulty in identifying the tips of the cervical transverse processes due to their shorter length. It is mainly given at the C6 or C7 vertebral level. The probe needs to be kept anterolaterally rather than posteriorly to see the cervical TPs [44]. It may not be safe due to its proximity to the neuraxis (shorter transverse processes) and the possibility of bilateral phrenic nerve involvement [45-48].
Thoracic ESPB at the upper vertebral levels (T2 orT3) can be preferred in cervical spine surgery by inserting the needle from caudal-to-cranial direction to achieve the desired LA spread and avoid technical difficulties and complications associated with cervical ESPB. Thoracic ESPB can provide multilevel analgesia even with the small volumes of LA due to rigid boundaries of the thoracic paravertebral spaces that facilitate drug spread at several levels involving ventral and dorsal rami. Lower thoracic level ESPB is mainly performed for lumbar spine surgeries by inserting the needle from cranial-to-caudal direction to achieve the desired LA spread and avoid technical difficulties associated with lumbar ESPB [49,50].
The lumbar ESPB can also be technically challenging due to the increased thickness of the ESMs with their tendinous attachment to the TPs [51, 52] and increased corresponding depth of the intermuscular plane in the lumbar region. The psoas muscle is also closely adherent to the vertebral bodies and the anterior surface of the TPs. The anterior drug spread to include ventral rami may be compromised due to the lack of clear boundaries of lumbar paravertebral spaces [53]. There is a communication through the fat-filled plane between the ESM and TP with the fat-filled psoas compartment containing lumbar nerve roots and plexuses. The spread of LA to the epidural space is possible through this communication [54]. The compressed lamina and the ligaments of the lumbar spine favor LA spread more into the epidural space [55, 56]. Thus, the lumbar ESPB may result in either lumbar plexus block or epidural anesthesia. The resultant weakness in the quadriceps or lower extremity muscles depends on the LA concentration and volume used in ESPB.
Sacral ESPB is mainly described for gender reassignment surgery or perineal surgery [57-61]. Its application for lower lumbar or sacral spine surgery is yet to be determined. The sacral multifidus plane block (SMPB), one of the variants of the paraspinal block, involves the deposition of LA in the plane under the multifidus muscle and bony area between the median and intermediate crests of the sacrum. The possible mechanism of action of SMPB includes blocking the dorsal rami and medial cluneal nerves directly by LA deposition and ventral rami by anterior LA spread through dorsal and ventral sacral foramina. The SMPB may also block the pudendal nerve (S2–S4), lumbosacral plexus, and sciatic nerve via the anterior and cranial LA spread [61, 62].
The role of LA
The possible role of the LA used in ESPB in causing postoperative neurological compromise depends on its inadvertent spread into either the epidural or subarachnoid space. It can be determined based on the occurrence and recovery pattern of the neurological symptoms. Distal-to-proximal and motor-before-sensory recovery patterns are the hallmarks of the differential blockade of the LA [23]. Inadvertent spread of LA into the subarachnoid space can lead to severe hypotension and bradycardia, resulting in unstable intraoperative hemodynamics. The consequences of the epidural spread depend on the density of LA around the spinal nerves, which could be compromised in a subsequent surgical dissection affecting the potentiality of the epidural space.
The concentration of LA, which determines the mass of the drug, also affects the efficacy of any block. The deliberate use of LA in low concentrations can result in a preferred motor-sparing analgesic effect of such high-volume blocks [63, 64]. Bupivacaine and ropivacaine are the most commonly used LAs for bilateral ESPB. Both LA agents consistently display preferential blockade of C-fibres (slow pain) > A-delta fibers (fast pain) > A-beta fibers (touch/pressure) in both preclinical and clinical studies [64-66]. With the increasing concentration, these agents may result in loss of proprioception and loss of motor function. Lipid solubility and higher pKa of LA facilitate intraneural diffusion and ion channel blockade. Ropivacaine exhibits a relative motor-sparing effect due to its lower lipid solubility than bupivacaine [67]. Twenty milliliters of 0.375% ropivacaine is recommended for each side of the bilateral ESPB in adults [68, 69].
Technical aspects of ESPB
Unexpected outcomes like a neurological compromise can be correlated with possible technical errors while administrating ESPB. The first technical aspect is identifying the correct landmark under ultrasound depending on the surgical extent and the desired level of the block. It may further depend on the sonoanatomy quality and the experience of the anesthetist. Sometimes misidentifying the lamina as the tip of the TP can lead to the retrolaminar block (RLB), another variant of the paraspinal block. In RLB, the needle insertion is slightly medial, targeting the lamina of the vertebra instead of the tip of the TP. It works via diffusion of LA into the paravertebral space through the soft tissue gaps between adjacent vertebrae [70]. Both RLB and ESPB were consistently associated with the posterior spread of injectate to the back muscles and fascial layers [37].
Fluoroscopic-guided ESPB can lead to RLB due to the inability to see the tip of TP clearly like under ultrasound, resulting in deposition of the LA solution over the lamina. The proximity of the RLB to the neuraxis can lead to a high probability of epidural spread, which carries the risk of motor weakness. The second important aspect is the ergonomics associated with bilateral ESPB. Administering the bilateral ESPB by standing on only one side of the patient may result in deviation from the ideal needle trajectory on one side compared to the other. Therefore, technical considerations should focus on stabilizing the needle by one person, injecting LA by another person, and performing such bilateral blocks while standing on either side.
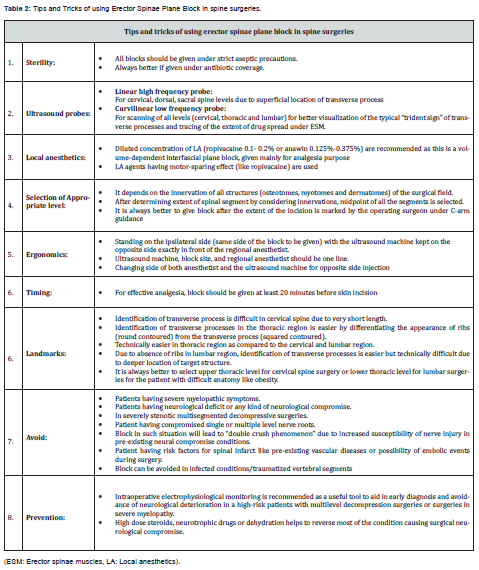
The third important aspect includes technical modifications such as keeping an ultrasound probe in a transverse view to help differentiate intramuscular drug spread from the effective linear drug spread between ESM and TP [71]. The fourth aspect is finding alternatives that involve dorsal rami consistently without causing drug spread to other unwanted areas. The thoracolumbar interfacial plane block is one such alternative that targets only the dorsal rami of the spinal nerve. Thus, it can provide more focused dermatomal coverage of the back required for thoracic and lumbar spine surgeries [72, 73]. However, its efficacy in spine surgeries is yet to be determined. We have suggested some tips and tricks for using ESPB in spine surgeries (Table 2), keeping all technical aspects in mind.
Conclusion
Postoperative neurological impairment following spine surgery is a serious concern for the operating surgeon and the patient. The role of ESPB in causing such complications is the diagnosis of exclusion made after a thorough evaluation of clinical symptoms and radiological studies. For that, understanding of various mechanisms involved in ESPB leading to neurological impairment is essential. It should encourage the anesthetists to take extreme precautions while administering this novel block, considering the anatomical differences at various spine levels. Surgeons should anticipate and explain the possibility of neurological deterioration while explaining the risks and benefits of the proposed surgical intervention. Intraoperatively, real-time neurophysiological monitoring is recommended as a useful tool to avoid further neurological deterioration, especially in extensive and multilevel surgeries or in high-risk and neurologically compromised patients.
After identifying or diagnosing such complications, intensive care and regular checking of spinal function are of great importance, along with simultaneous radiological workups to rule out various causative factors. Once paralysis occurs, early diagnosis and early intervention are essential in restoring spinal function. Despite the rare possibility of such complications, ESPB is still a promising option for ensuring effective perioperative analgesia in spine surgeries. It helps reduce postoperative morbidity by keeping the hemodynamic parameters stable and significantly reducing intraoperative blood loss. It can also avoid postoperative complications that lead to delay in mobility and discharge by significantly reducing the need for opioids and polypharmacy. However, further studies are needed to determine the safe concentration and volume of the LA solution used in ESPB, the exact surgery-specific vertebral level to cover desired surgical innervations, and the accurate LA deposition site to prevent spread to undesired areas.
To Know More About Journal of Head Neck & Spine Surgery Please click on:
https://juniperpublishers.com/jhnss/index.php
For more Open Access Journals in Juniper Publishers please click on:

Comments
Post a Comment