Biopsy-Proven Brain Metastases from Prostate Adenocarcinoma on 68Ga PSMA PET/CT: Case Series and Review of the Literature-Juniper publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF HEAD NECK & SPINE SURGERY
Abstract
Prostate cancer brain metastases are extremely rare
and typically occur at a late stage in the course of the disease with
poor prognosis. However, the incidence is rising as novel antiandrogens
and radionuclide therapy prolong survival and change the natural course
of the disease. Surveillance imaging of the brain is not the current
standard of care. We present two cases of patients who had brain
metastases from prostate adenocarcinoma initially detected on prostate
specific membrane antigen (PSMA) positron emission tomography/computed
tomography (PET/CT) and also provide a review of the epidemiology,
pathogenesis, imaging features, pathogenesis and current treatment
modalities of prostate cancer brain metastases. Our patient with
multiple brain metastases is still alive three and a half years post
initial diagnosis after being successfully treated with surgery,
androgen deprivation therapy and radiosurgery. This is the longest
survival time of any patient with multiple brain metastases and systemic
disease. We postulate that overall survival will increase with earlier
detection and treatment of brain metastases from a prostate cancer
primary and that scanning vertex to mid-thigh should be standard
practice with PSMA PET imaging.
Keywords: Brain metastasis; Prostate cancer; Neuroradiology; Ga68PSMA PET/CT
Abbrevations:
PCa: Prostate Cancer; Ga: Gallium; PSMA: Prostate Specific Membrane
Antigen; PET/CT: Positron Emission Tomography/Computed Tomography; MRI:
Magnetic Resonance Imaging; SRS: Stereotactic Radiosurgery; Gy: Gray;
PSA: Prostate Specific Antigen; PAP: Prostatic Acid Phosphatase; ADT:
Androgen Deprivation Therapy
Case 1
A neurologically intact 66-year-old man presented for
a Ga68 prostate specific membrane antigen (PSMA) positron emission
tomography (PET) scan due to a rising prostate specific antigen (PSA)
level of 7.4ng/ml thirteen years after a successful radical
prostatectomy for a Gleason 7 prostate cancer (PCa).
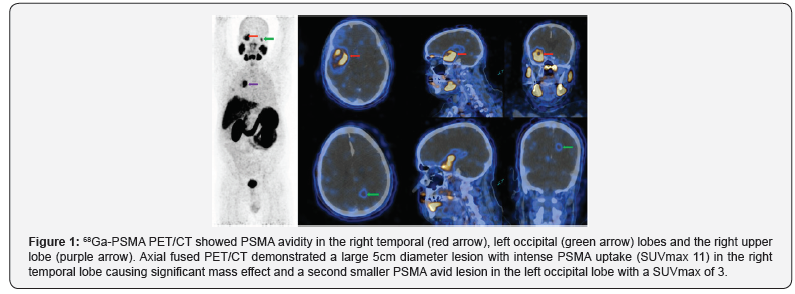
PSMA demonstrated an unusual distribution of disease with
intensely PSMA avid foci in an enlarged mediastinal lymph node,
within small lung nodules and in two PSMA avid brain foci within
the right temporal and left occipital lobes. Brain MRI revealed a
large 6.5cm complex enhancing lesion in the right temporal lobe
compressing the temporal horn and the body of the right lateral
ventricle. The cystic component of the mass showed no restricted
diffusion. A second 1.1cm lesion was noted in the left occipital
lobe superior to the ventricular trigone with peripherally
restricted diffusion and increased diffusibility centrally (Figure
1).
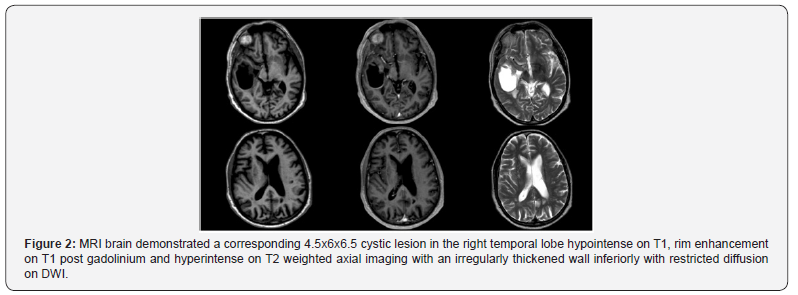
The patient underwent a right temporal craniotomy and
excision with gross total resection achieved (Figure 2). Histology
confirmed metastatic prostatic adenocarcinoma with positive
immunohistochemistry for PAP and PSA. The patient received
adjuvant stereotactic radiosurgery (SRS) to the right temporal
lobe cavity, left parietal lobe mass and hormonal manipulation
with androgen deprivation therapy (ADT).
Nine months postoperatively, the patient remained clinically
well with no neurological symptomology and undetectable PSA
with ongoing ADT. MRI brain confirmed no recurrence in the
right temporal lobe cavity but an increase in the size of the left
occipital lobe lesion. PSMA PET/CT demonstrated mild persistent
increased PSMA avidity in a solitary focus in the left occipital
lobe. Complete metabolic response at all other metastatic sites
with no new disease found. The patient was treated with further
SRS (14Gy in a single fraction) to the region of PSMA avidity in
the left occipital lobe. Three and a half years post operatively, he
remains well with good disease control.
Case 2
A 71-year-old man with metastatic castration resistant
prostate cancer previously treated with external beam
radiotherapy, ADT, radium 223 therapy and docetaxel presented
with a three-week history of increasing confusion and ataxia.
MRI brain revealed a well circumscribed 2.4 x 2.0cm
cystic, enhancing mass in the left para-median thalamus and
midbrain compressing the cerebral aqueduct and five other
lesions throughout the bilateral cerebellar hemispheres. The
thalamic cyst was biopsied and aspirated, but his symptoms
persisted. Histologic examination confirmed metastatic prostatic
adenocarcinoma. He was treated with whole-brain radiotherapy
but passed away two months following radiation treatment.
Intraparenchymal metastases from a prostate
adenocarcinoma primary is rare and only occur in an estimated
0.6-1.9% of patients [1-3]. However, the incidence is increasing
as novel antiandrogens and radionuclide therapy prolong
survival and change the natural course of the disease.
On the 1st of May 2018, a comprehensive literature search
examining peer-reviewed, English language articles from 1982
to 2018 was performed on multiple databases, yielding 1286
articles. These articles were reviewed and selected for studies
that met the following inclusion criteria;
a) Patients with intraparenchymal metastases from
prostate adenocarcinoma proven either on antemortem
resection or stereotactic biopsy and
b) Metastases confirmed on CT or MR brain imaging.
Additional relevant studies were also searched manually in
the reference lists of identified studies and by using the “related
articles” tool in PubMed. Patients with dural or skull-based
tumours extending into the brain were excluded from the review.
We found 1286 articles and considered 29 articles eligible. A total
of 47 men with brain metastases from prostate adenocarcinoma
origin confirmed on radiological imaging and antemortem
biopsy was thus identified in the literature (including our 2 cases
reported here).
Discussion
Epidemiology
For patients with brain parenchymal metastases from
prostate adenocarcinoma of any subtype, the mean at diagnosis
of brain metastasis was 66.3 years (range, 48-88 years) and the
median age was 66 years.
35 of the 47 men had known prostate adenocarcinoma at
the time of their cerebral metastasis diagnosis whilst for 12
men this was the initial presentation of their primary prostate
tumour. The mean age at diagnosis of brain metastasis for men
with known prostate cancer (PCa) was 65.5 years (range, 48-
88 years). In patients with cerebral metastases as the initial
presentation of their primary tumour, the mean age of brain
metastasis diagnosis was 66.4 years (range, 56-75 years).
Hatzoglou et al. [4] study of 7 patients with biopsy proven
brain metastasis from prostate adenocarcinoma was not
included as we were unable to differentiate the patients’ mean
age at diagnosis of brain metastasis from other patients in
their cohort who had biopsy from other sites of distant disease
confirming metastatic PCa.
Timing and symptoms
Patients with metastatic disease to the brain developed
disease an average of 4.4 years (range, 3 days to 13 years) after
the initial diagnosis of their PCa. New therapies increasing
overall survival time gives the tumour enough time to develop
brain metastases, that is usually a late event of the disease [5,6].
Neurological manifestations on presentation varied
according to the anatomical site with most metastases situated
in arterial border zones and the junction between the cortex
and subcortical white matter. The majority of patients (95.7%)
presented with overlapping neurological signs and symptoms.
22 patients presented with headache, the most common
presenting complaint at diagnosis followed by motor weakness
(n=16), ataxia (n=13), confusion (n=10), seizures (n=8), speech
impairment (n=7) and visual field disturbances(n=4). In the
two neurologically asymptomatic patients, the brain metastases
were diagnosed on surveillance imaging after a rise in PSA.
In 11 patients, the brain was the sole site of distant metastasis
[7-16]. In patients with metastatic disease, the most common
extra prostatic sites were bone (61.7%), lungs (26.4%), liver
(16.1%) and lymph nodes (13%).
Lesion characteristics
A total of 73 metastases was seen in the 47 patients. 39
patients (83%) had a solitary brain metastasis whereas the other
8 patients (17%) had multiple metastases. Four patients (8.5%)
had two metastases and 4 patients (8.5%) had 6 or more brain
metastases.
The frontal lobe (n=15) and the cerebellum (n=15) were
the most prevalent sites of metastases followed by the parietal
(n=14), temporal (n=12) and occipital (n=6) lobes. One patient
had a solitary metastasis in the temporo-parietal lobe and one
case involved the parieto-occipital lobe. Five patients had 6
metastases in the brainstem. Two cases involved the cavernous
sinus and one patient (our case) had a metastasis in the thalamus.
49 patients (67.1%) had parenchymal metastases located in
the cerebral hemispheres which corresponds to what is known
about the distribution of parenchymal metastases from other
primary cancers.
15 patients (20.5%) with brain metastases from PCa origin
were found in the cerebellum and only 8.2% of metastases
were found in the brainstem. This distribution of intracranial
metastases is similar to metastases from breast, lung and
melanoma where approximately 15% of metastases are found
in the cerebellum. Brainstem are uncommon sites especially
for solitary lesions and account for <1% of all brain metastases.
Interestingly 3 patients (4.1%) in our review of all the literature
to date had solitary metastases in the brainstem [8-9,17].
31 patients (68.9%) had one or more metastatic brain
lesions in the supratentorial compartment [7,9-13,16,18-25],
11 patients (24.4%) in the infratentorial compartment [8-
10,13-14,17,23,26] and 3 patients (6.7%) in both compartments
[27-29]. Two patients with lesions in the cavernous sinus were
excluded.
Intraparenchymal cerebral metastases from prostate
adenocarcinoma are rare and multiple metastases without
systemic disease is exceedingly uncommon. In the largest case
series to date of 16280 patients with brain metastases from
prostate cancer by Tremont-Lukats et al. [1] only 103 patients
(0.6%) had parenchymal brain metastases. In most cases the
metastases were singular (86%) and supratentorial (76%).
Only 3 patients (2.9%) of the cohort had both infratentorial and
supratentorial metastases.
A more recent case series by Hatzoglou et al. [4] found 10
patients (47.6%) of their cohort had both supratentorial and
infratentorial metastases. This is significantly higher than
other case series to date and is likely due to MRI images from
Hatzoglou’s case series being reviewed by a neuroradiologist
versus some cases in our review being limited to only single
slice images of patients’ CT or MRI which limits our ability to
ascertain the distribution of lesions outside of our two cases.
Gross pathology
Parenchymal metastases are generally round, discrete lesions.
The metastases in our review had variable peritumoural oedema,
necrosis and mass. Non-uniformity in the spatial distribution
of the parenchymal metastases suggests that vulnerability to
metastases differ according to its anatomical location. Our review
found parenchymal metastases from PCa had a predilection
for the frontal lobe and cerebellum (n=15, 20.5%) which is
consistent with other large case series which found 17-25% of
metastases from PCa were found in the cerebellum [1,4]. Unlike
melanoma, renal cell carcinoma and choriocarcinoma which are
particularly prone to developing intratumoural haemorrhages,
brain metastases from PCa was not found to have intratumoural
haemorrhage on imaging or histopathology. All of the patients
included in our review had biopsy confirmed adenocarcinoma
with positive immunohistochemical staining for PSA and PAP.
Imaging features
The cerebral metastases had highly variable imaging
appearance and was difficult to differentiate from metastases
originating from other primary tumour sites. Eight patients in our review only had CT imaging of their cerebral metastases
[7,9,18-21,26]. On non-contrast CT imaging, most metastases
were isodense to slightly hypodense relative to grey matter.
The majority of parenchymal metastases enhanced strongly
following contrast administration.
On axial T1-weighted MRI, most of the parenchymal lesions
were mildly hypointense. On post gadolinium imaging, nearly all
of the non-haemorrhagic metastases showed enhancement with
patterns of disease varying from solid uniform enhancement to
nodular or ring like lesions. FLAIR imaging also demonstrated
significant variability in lesion cellularity, presence of
haemorrhage and amount of peritumoural oedema. On diffusion
weighted imaging (DWI), well differentiated adenocarcinoma
metastases generally showed no diffusion restriction compared
to highly cellular metastases which demonstrated diffusion
restriction.
Cystic intraparenchymal metastases from PCa are rare with
only eight cases documented in literature to date, including our
two patients [11,19,22-23,27,30]. Five patients had solitary
cystic lesions and three patients had multiple cystic lesions.
Intralesional restricted diffusion was present in both our
patients.
Unlike true intraparenchymal cerebral metastases of
prostate adenocarcinoma origin, prostate cancer is the second
most common primary after breast cancer to metastasize to the
dura [5] and poses a radiological diagnostic challenge especially
when it presents as a solitary lesion which can be mistaken for a
meningioma as up to 44% of prostate metastases have a dural tail
[6,31,32]. Distinguishing between the two lesions is important
due to the poor prognosis of intracranial metastatic PCa and the
potential for conservative management versus active treatment
if a radiological diagnosis is made. The incidence of brain
metastases in the most recent series of 16,280 prostate cancer
patients is reported to be around 0.63% [1] which is less than the
1-2.4% incidence reported in autoptic series [3,33-34] with the
incidence of brain metastases being detected in the pre-MRI era
the same as in the post MRI era [1]. We postulate that advances
in MR imaging such as triple dose gadolinium and or 3.0Tesla (T)
MRIs have led to earlier detection of metastases in PCa patients
which allow earlier treatment and thus decrease the potential
for further extraprostatic spread.
Ga68 PSMA PET/CT imaging appears to have superseded F18
FDG PET/CT, CT and MR imaging not only in the staging of PCa
but also in the detection of PSMA-avid disease and is increasingly
being used for restaging recurrent PCa. Our case demonstrates
the importance of scanning from vertex to mid-thigh as albeit
rare, PCa can metastasize to the brain and earlier detection and
treatment correlates directly with improved survival time and
quality of life.
Treatment
All of the patients included in our review underwent
either surgical resection (n=37) or stereotactic biopsy (n=9)
of their intracranial lesion which confirmed their diagnosis
on histopathology. 31 patients (66%) underwent whole-brain
irradiation; 22 patients had adjuvant whole-brain radiotherapy
post-surgical resection of their lesion and 9 patients had
whole-brain radiotherapy as their primary treatment. Three
patients had stereotactic radiosurgery (SRS) to their lesion;
1 in conjunction with whole-brain radiotherapy post-surgical
resection and 2 patients had SRS post-surgical resection of the
main symptomatic lesion. 6 patients had trimodality treatment
with surgical resection, whole-brain radiotherapy and ADT
[10,13,18-19,24-26]. One patient had surgical resection of
the dominant metastasis, SRS to another metastasis and also
received concurrent androgen deprivation therapy (our case).
Surgical resection with adjuvant whole-brain radiotherapy
has been the Gold standard for treating solitary metastasis in the
brain. This combined strategy has been evaluated in randomised
controlled trials to significantly prolong survival, alleviate
neurological symptoms and reduce the risk of recurrence when
compared with surgical resection or whole-brain radiotherapy
alone [35].
ADT have also been found to bring both symptomatic and
radiological improvement [36-37] leading not only to an increase
in the overall survival time but also an improved quality of life
and is thus used in both the curative and palliative settings in
patients with prostate adenocarcinoma.
Prognosis
For the 41 patients who had documented survival times from
the initial detection of their brain metastases, the mean survival
time was 13.7 months (range, 3 days to 7 years). The patient
who had the longest survival time of 7 years in our series had a
solitary metastasis in the cerebellum that was resected en bloc
and also underwent a bilateral orchiectomy. Previous case series
have reported a median duration from diagnosis to death of
between 3.5 to 31 months [1,38-39]. Patients without systemic
disease were less likely to have brain lesions [4]. Patients with
brain metastasis as the sole site of extra-prostatic disease had
a mean overall survival time of 24.6 months (range, 1month to
7 years) compared to 13.4 months (range, 3 days to 5 years) in
patients with systemic disease.
We postulate that advances in imaging such as Ga68 PSMA
PET/CT, triple dose gadolinium and 3.0 Tesla (T) MRIs have led
to earlier detection of metastases in PCa patients which allow
the patient to be treated earlier thus decreasing the potential
for further extraprostatic spread and the increased incidence
of patients presenting with brain metastases as the sole site of
disease from their primary PCa.
Solitary brain metastasis has better prognosis than
patients
with multiple brain metastases. The overall survival time for
patients with a solitary brain metastasis was 14.3 compared
to 7.2 months (range, 3 days to 29 months) for patients with
multiple metastases. Our patient had the longest survival time
for a patient with multiple brain metastases of three years likely due
to low volume disease in the brain post-surgical resection
and exceptional response to ADT at the extracranial metastatic
sites.
The mean overall survival time for patients who had surgical
resection was 20.3months (range, 3 weeks to 7 years). The mean
overall survival time for patients who had stereotactic biopsies
was considerably shorter at 6.25 months (range, 1month to
2years). This latter cohort of patients either had disease in
the brainstem that was unresectable [8,17,21] or had multiple
cerebral metastases [13,16,28].
Prior to the introduction of docetaxel in 2002, the incidence
of prostatic brain metastasis from 1994 to 2002 was 0.8%. In the
post-docetaxel era (2002-2011), this incidence had increased to
2.8%. This represents a 239% increase in the frequency of brain
metastases from PCa between the two observation periods [40].
As the appearance of parenchymal metastases usually occurs in
the late phase of the disease process it appears that the increase
in frequency may actually reflect a gain in overall survival. In
general patients are living longer with brain metastases in the
context of PCa due to advances in imaging ability, systemic
treatment and increased surveillance.
Pathogenesis
PCa rarely metastasizes to the brain with the incidence in
large case series ranging from 0.63 to 1.1% [2,41] which suggests
that the brain parenchyma is resistant to the establishment of
metastatic foci by prostate carcinoma cells.
Currently the pathogenesis of cerebral metastases from
PCa is unknown however in summary there are two main
mechanisms postulated;
a) Single step spread via Batson’s paravertebral venous
plexus draining the prostate. Low pressure in the large
venous plexus allowing Valsalva maneuver to generate
enough pressure to reverse blood flow from the IVC to the
venous plexus, avoiding the lung and reaching the CNS.
This mechanism however does not explain the absence of
vertebral metastases and
b) Multi step haematogenous spread where secondary
seeding of tumour cells to the brain occur from a primary
metastatic focus involving the lungs or bones with brain
metastasis usually a late event in the course of prostate
cancer.
Haematogenous metastases have a special predilection for
arterial border zones and the junction between the cortex and
subcortical white matter. PSA is a sensitive indicator of the
presence of disease however serum levels of PSA did not correlate
to the development of brain metastases in our cases which is
consistent with what is found in other case series [11,22].
Conclusion
Intraparenchymal spread of prostate cancer should be
considered in men over the age of 60 years as a treatable
cause of gradual neurological deterioration especially if a
cranial malignancy or hyperostosis is found. The incidence of
intraparenchymal brain metastasis is only expected to increase
due to the longer life expectancy of patients with prostate
adenocarcinoma with novel therapies. Patients undergoing
Ga68-PSMA PET/CT for staging of PCa or when there is a PSA
rise should be scanned from vertex to mid-thigh as albeit rare,
prostate cancer brain metastases is a not to be missed differential
in this particular group of patients.
Consent
The patients provided informed consent to the publication of
their data, de-identified PET and MRI scans. No ethics approval
through an institutional committee on human research was
required.
To know more about Open Access Journal of
Head Neck & Spine Surgery please click on:
To know more about juniper publishers: https://juniperpublishers.business.site/

Comments
Post a Comment