Minimally Invasive Surgery for Adolescent Idiopathic Scoliosis: Anterior and Posterior Techniques-Juniper publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF HEAD NECK & SPINE SURGERY
Abstract
Purpose:Surgical gold standard for adolescent
idiopathic scoliosis (AIS) is open posterior spinal instrumentation and
fusion. This approach causes significant soft tissue disruption and
paravertebral muscle detachment. This review attempts to provide an
overview of the current state of knowledge of minimally invasive (MIS)
surgery for AIS.
Methods: The results of MIS for adult scoliosis
are briefly reviewed as the rationale for its application to AIS. A
review of the two currently available MIS techniques for AIS is then
given, including indications, surgical technique, and results in the
literature.
Results: Thoracoscopic anterior spinal fusion
and instrumentation (TASF) offers similar results and complication rates
with decreased blood loss but is technically demanding and has limited
indications. The posterior MIS technique seems to offer similar results
and complication rates, decreased blood loss and shorter length of
hospital stay, at the expense of a longer operative time.
Conclusion: Both TASF and posterior MIS are
valid alternatives to the gold standard. They have proven similar
results and short-term safety to open posterior fusion, with the added
theoretical advantage of a less traumatizing exposure and improved
cosmesis. While TASF has fallen out of favor due to its technical
challenges, specific instrumentation and limited indications, posterior
MIS does not suffer from these drawbacks. The limited amount of
literature on this technique warrants studies with further follow-up,
demonstrating its long-term safety before recommending its routine use.
Keywords: Adolescent
idiopathic scoliosis; Minimally invasive surgery; Posterior spinal
instrumented fusion; Thoracoscopic anterior spinal fusion;
Instrumentation
Adolescent idiopathic scoliosis (AIS) represents the
majority (51%) of the indications for scoliosis surgery at the pediatric
age [1]. Untreated, this pathology results in a normal life expectancy
[2,3], in productive and high-level functioning adults, with minimal
physical impairment other than decreased body satisfaction and back pain
[4]. Surgical treatment is usually indicated for major curves exceeding
40° in growing adolescents, or 45° in skeletally mature patients [5-8].
Its main purposes are to achieve a solid spinal fusion mass to prevent
curve progression and to obtain three-dimensional correction of the
deformity, while preserving balance in the coronal, sagittal, and axial
planes [9].
Operative treatment has drastically evolved over the
last century, from posterior Harrington instrumentation and casting,
which was associated with high failed fusion rates, poor deformity
correction, and the need for prolonged bed rest. Anterior thoracoscopic
and miniopen approaches were later developed, which resulted in
significant curve correction and high fusion rates [10]. Posterior
fusion with segmental
instrumentation, which is the current standard technique, has
led to an improvement of deformity correction in the frontal and
sagittal planes, together with a solid spinal arthrodesis and low
complication rate [11-13].
Despite reproducible good outcomes, the standard open
posterior approach requires extensive muscle dissection and
soft tissue disruption, and is consequently associated with
significant blood loss, postoperative pain, prolonged recovery,
and a long midline scar [12,14]. In an effort to decrease this
approach-related morbidity, a minimally invasive surgery (MIS)
technique for posterior spinal fusion has been recently proposed
for AIS [15]. Although promising, this posterior MIS technique for
the treatment of adolescent deformity is far from being widely
recognized, with currently very limited evidence to support its
routine use [14].
This review aims to provide an overview of the current state
of knowledge of MIS for AIS. We first briefly review the results of
MIS for adult idiopathic and degenerative scoliosis, which provide
the rationale for its application to the pediatric population. We
then describe the indications, surgical technique, and results of
thoracoscopic anterior spinal fusion and instrumentation (TASF),
which is currently the only MIS alternative to the posterior MIS
technique for AIS, despite its fading out. A detailed review of
the posterior MIS technique for AIS is then provided, including
technique, indications, and results in the current literature.
Surgery for adult spinal deformity is a challenging procedure,
due to a combination of patient- and intervention-related factors.
Patients have medical comorbidities, physical deconditioning due
to pain and bed rest, decreased bone mineral density, a stiff spine
deformity, and distorted vertebral anatomy. The surgery itself is
extensive, involving long spine fusion with instrumentation and
osteotomies, and most often interbody fusion or an anterior
approach to obtain a 360° fusion [16]. Unsurprisingly, reported
complication rates are significant, even in experienced hands,
with 52% of patients affected by a perioperative complication
(6 weeks), 43% by a delayed complication (2 years) [17], and
a postoperative mortality (30 days) of 2.4% [18]. Therefore,
the safety of adult spine deformity surgery represents a major
challenge, and the modern MIS technique has emerged in an
attempt to lessen its morbidity in the aging population.
To date, most MIS techniques for degenerative and adult
idiopathic scoliosis have combined a lateral interbody fusion,
either with posterior percutaneous pedicle screw insertion in
so-called circumferential MIS (cMIS), or with traditional open
posterior surgery in so-called hybrid surgery [19-27]. These less
invasive techniques have proven useful for adult spine deformity,
with reduced complication rates compared to conventional open
techniques, but also appear to be less effective for the correction
of severe and/or fixed sagittal and coronal plane deformities
[28].
Adolescent spine deformity is generally considered less rigid
than adult and is thus theoretically more amenable to standalone
MIS techniques. The pediatric spine, because of its inherent
flexibility and ability to fuse, is theoretically ideal for MIS.
Given the positive results obtained with MIS to treat adult spine
pathologies, including deformities, the next logical step is the
application of less invasive surgical techniques to the treatment
of spine deformity at the pediatric age, AIS in particular.
During the nineties, thoracoscopic approaches were
increasingly used in spine surgery [29-31]. The first
thoracoscopic instrumentation and fusion for idiopathic scoliosis
was performed in October 1996 [30,32]. The main thoracic curve
pattern, Lenke type 1, is the most common in operative AIS
[33,34]. Although posterior spinal segmental instrumentation
and fusion is considered the gold standard, open anterior
surgery (thoracotomy) is associated with improved sagittal
correction and saving of an average of 2.5 to 4 distal lumbar levels
in prospective studies [9,35]. Additionally, the diskectomies
performed in anterior correction of thoracic curves theoretically
allow for better coronal correction [29], though two prospective
studies found no significant difference in major curve correction
compared to posterior surgery [9,35]. The main disadvantages
of open anterior fusion are diminished postoperative pulmonary
function, which persists at 2 years follow-up, and transient
shoulder girdle dysfunction [9,36-38]. A few centers have
contributed to develop a thoracoscopic technique for anterior
instrumentation and fusion, in an effort to minimize the anterior
approach-related morbidity. These techniques have indeed been
shown to have less effect on pulmonary and shoulder girdle
function postoperatively [36-40].
Preoperative planning and patient selection
TASF for AIS is indicated in Lenke type 1 (main thoracic)
curves [29,34] that require at most 8 segments of fusion
within the boundaries of T4 and L1 [12,41]. While the Cobb
end vertebrae are usually chosen as limits for fusion, the lower
end vertebra can be excluded if the one above it is neutral [29].
Fusion down to L3 is achievable with an added mini-open
retroperitoneal approach [30]. On AP radiograph, curves should
measure up to 70°, and be flexible to at least 50% or reduced to
30° on ipsilateral bending [12,29,30,41]. On lateral view, thoracic
kyphosis (T5-T12) should be no greater than 40° [41], since it is
increased by the discectomies and compression technique used
for reduction [9,12,29]. The ideal candidate is slender (40-60kg),
for easier portal placement and use, and tall, for ample space in
the chest cavity and larger vertebral bodies for screw insertion
[29].
Given the need for single-lung ventilation, pulmonary
function testing is mandatory [12,29]. Poor pulmonary function,
previous ipsilateral thoracic surgery, recurrent pneumonia and
pulmonary tuberculosis are contraindications, as are osteopenia, seizure disorders and inability of the patient to comply with
postoperative instructions due to the risk of implant loosening
with the single-rod construct [12].
Surgical technique
A thoracotomy tray must be available and open, and
the surgeon ready to perform an emergency thoracotomy
if mandatory, because some complications such as massive
bleeding can be difficult to treat through a thoracoscopic access
[31].
Five or six portals are used, depending on the number of
levels to be instrumented: three or four posterior portals for
instrumentation and two anterior portals for scope insertion,
spine exposure and discectomies. Once the pleura has been
dissected to expose the spine circumferentially, the vertebral
discs are removed, and the resulting spaces packed with
hemostatic agent. Vertebral screws are inserted at a 10° angle
away from the spinal cord, purchasing both cortices, and
accurate placement is confirmed with fluoroscopy. A stainlesssteel
rod is cut to the appropriate length, measured with a balltip
cable run through the screw heads. Bone graft is introduced
between the endplates. The rod is then inserted successively
over each screw head, applying compression with the next screw
head at each segment before tightening the rod in place. A chest
tube is inserted, and final X-rays are obtained to confirm curve
correction and correct instrumentation [12].
Several types of bone graft may be used: rib autograft alone
or augmented with demineralized bone matrix in a putty form,
or morselized iliac crest autograft. A fibular strut or femoral ring
allograft can also be added at the one or two distal levels of fusion
to prevent excessive kyphosis in lower segments [12,30,42].
The chest tube is typically removed on the second or third
postoperative day. A custom-molded clamshell thoracolumbar
orthosis (TLSO) is prescribed for 3 months postoperatively when
the patient is out of bed [12,30,32,42]. Sports, bending and lifting
activities are authorized after 3 to 6 months or radiographic
evidence of solid anterior fusion [12].
Results
Initial results with TASF for AIS have been encouraging,
despite a significant learning curve.
Compared to the standard posterior approach with pedicle
screw fixation, TASF has similar radiographic results, patientbased
clinical outcomes and complications rates in Lenke 1
curves up to 70° with a normal or hypokyphotic spine. Advantages
include decreased blood loss and transfusion rate, reduced
total incision length, fewer fused levels, and preservation of
approximately one distal level. Disadvantages include prolonged
operative time, the need for a chest tube postoperatively and
TLSO brace during 3 months, and slightly lesser improvement of
pulmonary function [9,41,43].
Patients are back to school after 2 to 4 weeks and can
discontinue pain medication before 4 weeks [30]. Complications
of TASF are primarily due to instrumentation failure (rod
breakage), pseudarthrosis and pulmonary issues. Interestingly,
deep wound infections are virtually non-existent after TASF,
contrary to posterior fusion [9,30,32,37,41,43-49].
A steep learning curve of 28 [46] or 30 [50] procedures has
been described, during which deformity correction significantly
increases and operative time significantly decreases. There does
not, however, seem to be a significant difference in complication
rates or blood loss throughout the operator’s experience.
Moreover, convening an experienced access surgeon may
decrease this learning curve [31]
TASF is therefore a valid MIS option for AIS. This technically
demanding procedure, with an acceptable complication rate
including during the learning curve, is however restricted for
thoracic curves (Lenke 1) with a normal or hypokyphoticspine
and offers similar results for these patients when compared to
the gold standard that is open posterior spine fusion. However,
due to an increased degree of correction and lower risk of
instrumentation failure associated with the modern posterior
technique, most spine surgeons, including the proponents of
this technique, have converted almost entirely to the posterior
approach for thoracic AIS. Those still using TASF generally restrict
it to patients with specific concerns regarding the appearance of
a long posterior midline scar, and who would accept wearing a
postoperative brace [43,44]. Hence a posterior MIS technique,
with a wider range of indications and simplified instrumentation,
could be more largely accepted by spine surgeons.
The current gold standard for the surgical treatment of AIS
is open posterior spinal instrumentation and fusion [9,12,41].
This approach creates a long midline incision spanning the
vertebral levels to be fused. The appearance of the surgical scar
seems to be an important factor in the patient’s perception of the
outcome [50,51]. In addition, this approach requires extensive
detachment of most of the paravertebral muscle insertions from
the spinous processes medially to the transverse processes
laterally. This causes long-lasting paravertebral muscle injury
and denervation, leading to persistent atrophy and decreased
strength of trunk extension [52-60]. These injuries are related to
retraction pressure, time, and extent of exposure [61].
Given the positive results of MIS techniques in adult spine
pathologies, including deformities, the next logical step is to
apply them to pediatric deformity surgery, AIS in particular. Yet
many technical challenges are faced in this patient population
[15,62].
In contrast to adult degenerative scoliosis, AIS patients
present with larger curves (≥45°), consequently more levels instrument (7 to 13), three-dimensional deformity and usually
significant vertebral rotation. In cases with double or triple
major structural curves (Lenke 2, 3, 4 and 6), passing a rod with
normal sagittal contour may be laborious. While the insertion
of multiple percutaneous pedicle screws through separate stab
incisions is routine in the adult patient population, this is not an
option in AIS patients for multiple reasons. First, 14 to 26 screws
would need to be inserted under fluoroscopic guidance, thereby
increasing radiation exposure to both the surgeon and the
young patient. The multitude of incisions would also probably
be a major cosmetic concern in adolescents. Finally, surgical
access would be a concern due to the small size of the incisions.
Performing adequate facetectomy would be difficult, though
crucial for fusion. The multiple reduction maneuvers required
for the large deformities encountered in AIS (rod translation, rod
de-rotation, in situ bending, direct vertebral rotation, and spine
translation) would be limited by both the incision size and MIS
instrumentation systems [15,62].
In this setting, a specific MIS technique for AIS has been used
since May 2008, based on three small cutaneous incisions with
an underlying muscle splitting approach. It has been developed
with the aim to limit incision length and soft tissue dissection,
while allowing large facet osteotomies to promote fusion,
insertion of pedicle screws with a free hand technique, and
effortless introduction of contoured rods [15].
Surgical technique
The patient is installed prone on a radiolucent table, as for
a conventional posterior approach. After prepping and draping,
fluoroscopy is used to determine the length and localization of 3
separated midline skin incisions (Figure 1). Fluoroscopy use is
limited to the planning of the skin incisions [14].
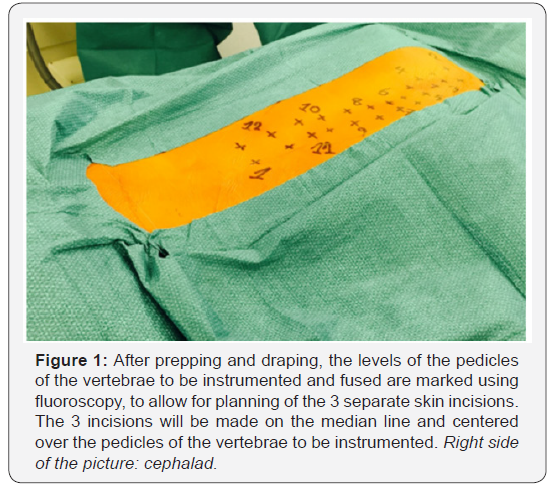
The skin is then undermined laterally to allow for
paramedian extraperiosteal fascial incisions on each side of
the spine. A blunt muscle sparing approach is used down to
the facet joints in the lumbar spine, and to the transverse
processes in the thoracic spine. This is similar to the paraspinal
sacrospinalis-splitting approach described by Wiltse for the
lumbar spine [63-65]. Posterior elements are uncovered using
electrocoagulation, from the basis of the lamina to the transverse
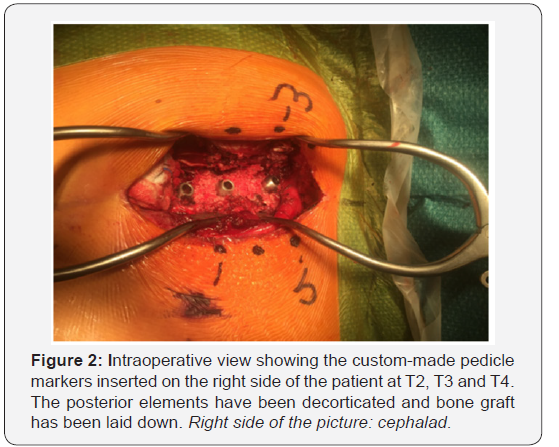
processes. Wide facetectomies are performed. The pedicles are
cannulated using the free hand technique [66] and marked with
pedicle markers. The facet joints are then decorticated using
a high-speed burr. Bone graft (a mixture of autograft from the
facetectomies and freeze-dried allograft) is applied before
definitive instrumentation (Figure 2).
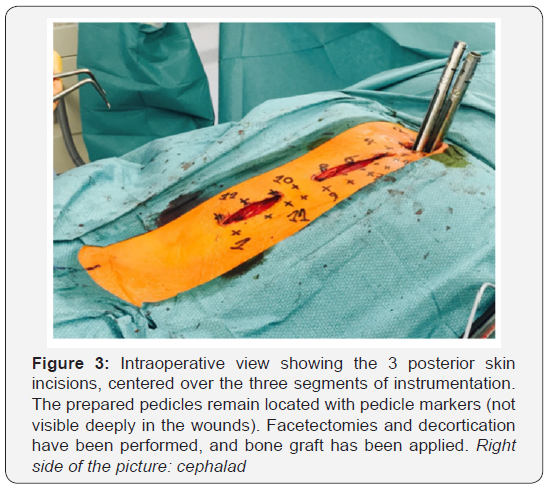
Three segments are instrumented, one per skin incision
(Figure 3). The facet joints located between skin incisions are
not instrumented, but facetectomies, decortications and bone
grafting are still performed by mobilizing the skin and soft
tissues.
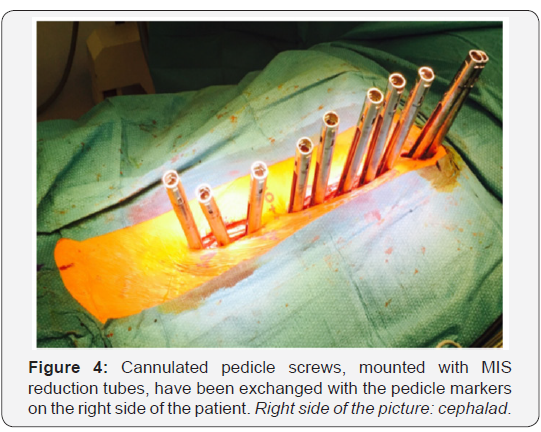
Cannulated pedicle screws, already connected to
reduction
tubes, are then exchanged with the cannulated pedicle markers
using a guide wire (Figure 4). Monoaxial screws are placed in the
convexity of the major curve. The remaining screws are polyaxial.
Some alternate reduction screws with extended tabs and MIS
screws with open connectors (reduction tubes) between levels
[15,62]. Others [67] favor MIS screws with reduction tubes at
every instrumented level, in order to facilitate insertion of the
rod and apical derotation during deformity reduction.
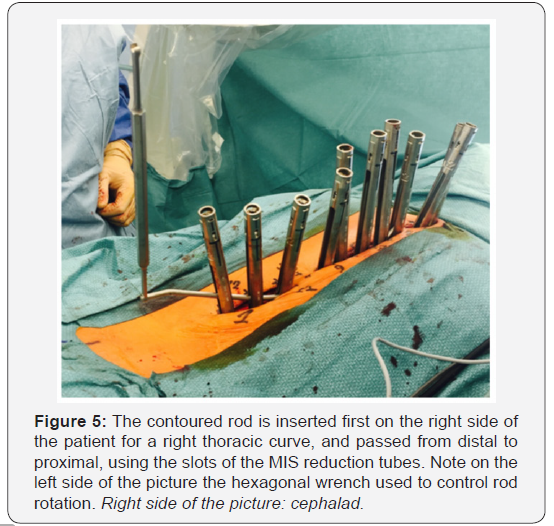
Two 5.5mm cobalt chrome rods are cut to the measured
length and contoured with the appropriate sagittal profile. The
convex side rod is introduced first. It is passed below the fascia
of the cutaneous bridge under direct visualization, then through
the slots of the reduction tubes. Inserting the rod from caudal to
cephalad avoids conflict with the patient’s head (Figure 5), but
some argue that the opposite direction offers increased safety
due to the fact that the orientation of the overlapping laminae
and facets in the thoracic spine tends to prevent accidental
penetration of the spinal canal [15,62]. Rod derotation and a
gradual spine-to-rod reduction technique are used to correct
most of the deformity. The rod is secured using set screws, and
direct apical segmental derotation is performed. The pedicle
markers on the concave side are finally exchanged for pedicle
screws. The second concave rod is overcontoured in the sagittal
plane to allow for further deformity correction in the transverse
plane.It is then also inserted from distal to proximal.
Results in the current lite
There is currently limited data on the application of posterior
MIS in pediatric spine deformity, despite the fact that it may have
the potential to significantly improve perioperative morbidity
Samdani et al. [68] first reported their results using posterior
MIS technique to treat AIS patients. Their surgical technique
varies slightly with the one previously described by the fact that
they used a single midline skin incision instead of 3 separated
incisions. This allows for increased implant density with bilateral
pedicle screws at every level fused. Fifteen patients were
operated with a mean age of 14.1 years (11-16) and an average
follow-up of 8 months (2-35). The preoperative major curve
Cobb angle was on an average 54° (45-82) and was corrected
to a mean of 18° (9-35). This represents an average correction
of 67%. In the sagittal plane, thoracic kyphosis decreased from
31° (18-47) to 26° (15-34). The mean estimated blood loss (EBL)
was 254ml (100-672) and the average operative time (ORT) was
470 minutes (236-662). Regarding complications, one patient
presented with a pullout of the proximal screws at 8 months
postoperatively and required revision.
Miyanji et al. [14] reported their results comparing a
three-incision posterior MIS technique with standard open
posterior surgery for AIS. Sixteen patients treated with MIS
were matched for age, sex, curve type and size to 16 patients
treated with conventional open surgery. There was no significant
difference between MIS and open surgery in terms of major
Cobb angle correction (63% ±13 versus 68% ±8 respectively)
or postoperative thoracic kyphosis (21° ±9 versus 17°±5
respectively). In the MIS group, EBL was significantly lower
(277ml ±105 versus 388ml ±158), ORT was significantly longer
(444min ±89 versus 350min ±76) and hospital stay (LOS) was
significantly shorter (4.63 days ±0.96 versus 6.19 days ±1.68)
when compared to open surgery.
In a retrospective comparative study, Sarwahi et al. [62]
compared 7 MIS patients to 15 open posterior patients with
2 years of follow-up data. MIS was associated with a longer
median ORT despite a decreased implant density. There was no
significant difference in EBL, EBL per fixation point, amount of
fluids administered, number of intensive care unit days, LOS, time
to mobilization, visual analog pain scores, patient-controlled
analgesia days, deformity correction, screw placement accuracy,
fusion rate or complications. There was a lower incidence of
postoperative anemia and transfusion in the MIS group. The
difference in ORT should be interpreted with caution, given the
fact that this small series of 7 patients was considered the senior surgeon’s learning curve. Considering what we know from TASF
[45,49] and another series on posterior MIS [67], this most likely
represents the very beginning of the learning curve.
It is worth noting that LOS is influenced by numerous
factors, among which postoperative management seems to
play an important role. Postoperative admission of AIS patients
to a general ward, rather than an intensive care unit, has
been correlated with a reduced LOS and decreased analgesic
medication usage [69]. The use of an early discharge pathway has
been associated with a 48% shorter LOS after posterior spinal
fusion for AIS [70-72]. The two comparative studies mentioned
above did not specifically describe their recovery protocol, nor
did they specify whether postoperative management was any
different between MIS and open surgery groups [14,62].
The largest series to date is a retrospective analysis on
prospectively collected data by de Bodman et al. [67]. It reported
results from the first 70 consecutive patients who underwent
posterior MIS for AIS by a single surgeon, with a mean age of 15
years ±4.5 and a male to female ratio of 8:62. Preoperative major
Cobb angle averaged 58.9°±12.6 and was significantly corrected
to 17.7° ±10.2 (a 69% ±20 curve correction). Thoracic kyphosis,
as evaluated between T2-T12 and T5-T12, was also significantly
improved by 18% and 24% respectively.
Perioperative (30 days) complication rate was 4.3%, with 3
complications in 3 patients: 1 subcutaneous hematoma, 1 deep
venous thrombosis, and 1 pneumothorax. Five (7.1%) additional
complications occurred in 5 patients at an overall mean followup
of 2 years ±1.4: 1 superficial wound infection, 1 suture
granuloma, and 3 delayed deep surgical site infections (4.3%).
These 3 delayed deep surgical site infections were encountered
among the first 19 patients treated with MIS.
ORT averaged 337min ±121. ORT per level showed a steep
decrease over the first 25 cases, then stabilized to a plateau of
25min ±5. The mean EBL was 346ml ±175, and EBL per level
was 36ml ±14. No allogeneic blood transfusions were needed.
EBL also significantly decreased with increasing experience with
the surgical technique. The average LOS was 4.6 days ±0.8. The
average number of screws used was 18.6 ±4.1. The mean number
of levels fused was 11.2 ± 4.9, representing an overall average of
30 ±15.1 minutes of ORT per level.
These results indicate that MIS for AIS is associated with a
significant correction of spine deformity in frontal and sagittal
planes, together with low EBL and short LOS. The perioperative
complication rate compares well with the standard open
technique, as reported by large morbidity databases [1]. The rate
of delayed surgical site infection, however, was unusually high,
with reported rates after open posterior surgery ranging from
0.9% to 3% [1,73-76]. The duration of surgery is significantly
correlated with the rate of multiple complications [77]. Given the
reduction in ORT over the first 25 cases in this series, the high
rate of delayed infection could be due to a learning curve effect.
Taken together, the limited available data suggest that the use
of posterior MIS for AIS is associated with deformity correction
and complication rates similar to open posterior spinal fusion,
with potential benefits related to a less traumatizing exposure,
low blood loss and a diminished length of hospital stay. However,
these encouraging results are obtained at the expense of an
increased surgical time, which could be related, at least partially,
to a learning curve effect.
Posterior MIS for AIS is associated with low blood loss and
decreased length of hospital stay, while offering similar power
of deformity correction in the frontal and sagittal planes, when
compared with standard open posterior technique. However,
operative time is significantly increased, at least during the
surgeon’s learning curve. The longer-term safety of this
procedure needs to be documented in larger studies with a
minimum of 2 and 5 years of follow-up, before recommending its
routine use. Finally, the most important challenge will probably
be to offer this type of surgery to the frailest pediatric spine
deformity patients, without further increasing the significant
complication rate associated with the surgical treatment of
neuromuscular scoliosis.
To know more about Open Access Journal of
Head Neck & Spine Surgery please click on:
To know more about juniper publishers: https://juniperpublishers.business.site/
Comments
Post a Comment