Sequential Delivery of Growth Factors from Hydrolytically Degradable Silica-Based Nanoparticles for Cartilage Tissue Engineering-Juniper publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF HEAD NECK & SPINE SURGERY
Abstract
Articular cartilage shows a very limited self-healing
capability due to it's a vascular structure. It has been reported that
sequential supplementation of various growth factors such as bone
morphogenic protein 7 (BMP-7), transforming growth factor-beta 1
(TGF-01) and insulin-like growth factor-I (IGF-I) play critical roles in
reconstruction of articular cartilage tissue. The objective of this
work is to design a drug delivery system which facilitates the
controlled sequential delivery growth factors to maximize the extent of
chondrogenic differentiation of mesenchymal stem cells and consequently
reconstruction of damaged cartilage tissue. Surface-modified silica
nanoaprticles (SNs) with short segments of lactide (L) and glycolide (G)
units were used for grafting and timed-release of growth factors. It
was shown that by changing the length of L and G units, the release rate
of grafted bovine serum albumin (BSA) on the surface of SNs can be
controlled. Nanoparticles with bigger G units showed faster release rate
of BSA compared with shorter G units. Additionally, the presence of
short LG segments did not significantly change the size distribution of
SNs.
Articular cartilage can resist a significant amount
of mechanical stress and provide a lubricating surface for the gliding
joints and a load-bearing matrix attached to the underlying bone [1]
. However, due to its avascular nature and low metabolism, it has a
very limited self-repair capability upon suffering a trauma [2]
. The disease control and prevention center reported that nearly 27
million Americans suffer from joint pain and stiffness, loss of function
and disability [3].
While various strategies such as transplantation of autogenous or
allogenous chondrocytes, or the use of mesenchymal stem cells (MSCs) are
currently used for cartilage treatment, these strategies always suffer
from inherent risks of an immune reaction, lack of suitable donor site,
and more importantly they rarely restore the full function to the joint [4,5].
It has been demonstrated that different types of
growth factors such as bone morphogenic protein 7 (BMP-7), transforming
growth factor-beta 1 (TGF-β1) and insulin-like growth factor-I (IGF-I)
play critical roles in tissue engineering of articular cartilage to
induce chondrogenic differentiation of Mesenchymal Stem Cells (MSCs) [6-8]. However, the bioavailability and bioactivity of these growth factors are both time and concentration dependent [4,9,10].
Several studies have shown that the sequential supplementation of
growth these factors is critical to prevent dedifferentiation of cells
by first promoting proliferation with one specific growth factor, and
then differentiation and expression of a desired phenotype with another [9,10].
A critical barrier to progress in this way is the lack of suitable
delivery systems which work for precise controlled and orchestrated
time-dependent delivery of multiple growth factors, although many
studies have been conducted to control the delivery of growth factors
for different tissue engineering applications. Accordingly, the main
challenge of this work is to engineer a programmable delivery system to
control the release rate of BMP-7, TGF-β1 and IGF-I growth factors and
consequently maximize the chondrogenic differentiation of MSCs.
To address this challenge, the idea is to engineer a
silica based nanoparticle system containing short segments of
biodegradable polymers such as polylactic acid and polyglycolic acid to
control the release rate of grated proteins (Figure 1). We have chosen MSNs given their demonstrated biocompatibility, osteogenic potential[11] , and efficacy as drug delivery vehicles for sustained release of antibiotics [12] and anti-cancer drugs [13].
We envision that due to the hydrophilic nature of silica nanoparticles,
bioactivity of the grafted proteins will be significantly enhanced over
solid hydrophobic micro/nanoparticles such as poly lactic-co- glycolic
acid (PLGA) which are currently used for drug delivery applications.
Accordingly, we assume that the protein release rate can be tuned by
type or length of the degradable segments. The novelty of this project
is to design a low-cost hydrolytically degradable nanocarrier system
which facilitates a programmable sequential delivery of multiple growth
factors with a controlled timed-release to enhance chondrogenic
differentiation of MSCs for cartilage tissue engineering applications.

Materials: Lactide (L) and glycolide (G)
monomers with >99.5% purity (Ortec, Easley, SC) were dried under
vacuum at 40°C for at least 12h before use. N, N/-disuccinimidyl
carbonate (DSC) and bovine serum albumin (BSA) were received from
Novabiochem (EMD Biosciences, San Diego, CA) and Jackson Immuno Research
(West Grove, PA), respectively. Hydrophilic silica nanoparticles (SN)
was kindly donated by Evonik Corporation (New Jersy). All other reagents
were purchased from Sigma Aldrich (St. Louis, MO).
Methods: To activate the silanol groups of SNs
were activated by adding 1.5x10-4 mol triethyl amine (TEA) to 250mg of
SN containing 3x10-4 mol SiOH groups in 70ml toluene as the solvent. In
the next step, 6x10-4 mol of isopropyl alcohol was added to the mixture
to continue the reaction for 2h at 50 °C. Then, the desired amount of L
was added to the mixture along with 1ml tin (II) 2-ethylhexanoate as the
reaction catalyst and the reaction was run for 6h. The lactide
chain-extended silica was used as an initiator for chain extension with G
monomer with a predetermined L to G ratio. The reaction was allowed to
proceed for 6h at 50°C and the product was precipitated in ice-cold
hexane to remove the unreacted monomers. In separate reactions, the mole
ratio of L and G was changed from 100% (L100) to 75% (L75/G25) and 50%
(L50/G50) while the total amount of L and G was kept fixed at 3x10-3
mol. The synthesized copolymer on SNs surface was functionalized with
succinimide groups by reacting hydroxyl end-groups of the copolymer with
DSC as we described previously [14]. The product was purified by dialysis against DI water and lyophilized.
To attach BSA on the surface of modified SNs, 10mg
SNs was suspended in 0.5mL PBS by sonication for 1 min. Next, 0.5mL of
the protein in PBS (20mg/mL for BSA) was added to the SNs suspension.
The amine group of the protein was allowed to react with succinimide
end-groups of LG in the surface of SNs under ambient conditions for 12h
as we previously described (Figure 1) [15].
The protein grafted SNs were freeze-dried to obtain a free-flowing
powder. To determine grafting efficiency, the protein grafted SNs were
resuspended in PBS and centrifuged at 18,000 rcf for 10min and the
supernatant was analyzed for total protein content with the ninhydrin
reagent as we described previously [16].
Grafting efficiency was determined by dividing the amount of attached
protein (total - free protein) by the initial amount in the grafting
reaction.
Size distribution of the SNs was measured by dynamic
light scattering with a Submicron Particle Sizer (Model 370, NICOMP,
Santa Barbara, CA) as described previously [16,17]. For measurement of release kinetic, 1mg protein grafted SNs were incubated in 1mL PBS at 37°C as we previously described [15].
At each time point, the suspension was centrifuged at 18350 rcf for
10min, the supernatant was removed, the SNs were resuspended in 1mL
fresh PBS and incubated until the next time point. The amount of BSA in
the supernatant was measured with the ninhydrin reagent as described [16,18].
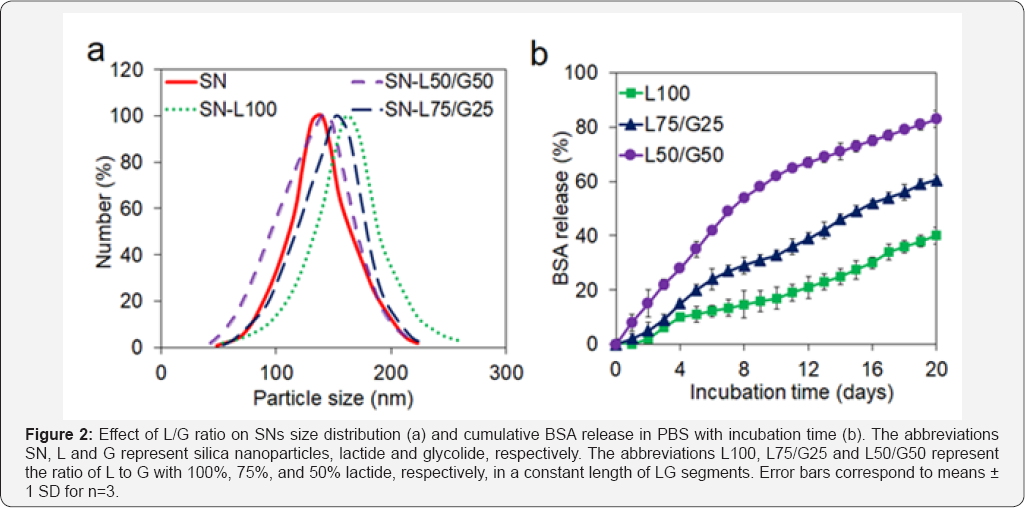
The calculated grafting efficiency of BSA to SNs
based on the procedure explained in the method section was 52±9%. The
effect of L and G segments on average diameter and size distribution of
nanoparticles are shown in (Figure 2a).
It can be observed the addition of short L and G parts in SNs does not
significantly change the average diameter of particles. The particle
sizes are between 120±12 and 165±15 for SN and SN- L100, respectively. Figure 2b
reveals the effect of the length of L and G segments on release rate of
BSA from nanoparticles. The results demonstrated that by increasing the
length of lactide the release rate of BSA will decrease significantly.
The cumulative release percentage of BSA is 51±5, 65±8 and 91±4 for L50/
G50, L75/G25 and L100, respectively, after 24 days. The average release
rate of BSA from L50/G50, L75/G2 5 and L100 is 3.2, 2.4 and 2.1 wt%,
respectively, during the first 24 days.
It has been reported that the release kinetic of BSA
grafted to the PLG copolymer follows the degradation rate of the
copolymer [19].
Additionally, it has been previously shown that the degradation kinetic
of LG based micelles depends on the proximity of water molecules to L
and G ester groups [20,21], which is dependent on hydrophobicity of the degradable units [19].
Therefore, the fraction of less hydrophobic G in LG segments has a
profound effect on LG unit degradation and consequently the release rate
of protein. As a result, by increasing the fraction of G, which is less
hydrophobic than L, from 0 to 50%, the the average release rate of BSA
increased from 2.1 wt% to 3.2 wt%.
It was shown that by surface modification of silica
nanoparticles with short segments of lactide and glylcolide, the
controlled release of growth factors can be achieved. Based on the
release profile of BSA from surface modified silica nanopartilces, by
increasing the ratio of glycolide segment from 0% to 50% with respect to
lactide segment, the average release rate of BSA will increase from 2.1
wt% to 3.2 wt% per day. Some of the potential challenges of using
nanoparticulate systems for drug delivery applications are the stability
of the nanoparticles in aqueous environment, bioactivity of growth
factors and controlling release mechanism of growth factors from
nanoparticles. Therefore, further studies need to be done on the
properties of surface modified nanoparticles as well as controlled
release of target growth factors like of BMP-7, TGF-^1 and IGF-Iand
their effect on chondrogenic differentiation of MSCs.
To know more about Open Access Journal of
Head Neck & Spine Surgery please click on:
To know more about Open access Journals
Publishers please click on : Juniper Publishers
Comments
Post a Comment