Did Randomized Clinical Trials (RCT) and Continually are Contributing Evidence for Progress in Radiation Oncology-Juniper publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF HEAD NECK & SPINE SURGERY
Abstract
Since clinical trials have been advocated as evidence
based guidelines for radiotherapy many RCTs were carried out. Can they
be considered compared with empirical studies, as a milestone progress
in radiation oncology. Large heterogenous tumor sites and stages were
enrolled into the trials on hyperoxygen therapy, radiosensitizers and
altered fractionations raise some uncertainties and criticism regarding
therapeutic gain usually reported as median end-points. Local tumor
control rates have been and still are related to the tumor TNM status,
but almost never to initial tumor volume (number of initial cancer stem
cells) whereas the effect of irradiation is cell killing, not tumor
stage killing. Many RCTs became disappointing or at least therapeutic
gain has been lower than expected. Well known trials are reviewed and
discussed. In contrary, some retrospective studies have provided
important practical information’s, i.g. tumor volume is more predictive
to design dose fractionation than T stage, overall treatment time has
been show as a strong determinant of treatment outcome. This finding
initiated series of altered fractionation trials. Alpha/beta values for
H&N tumors and cell survival curves derived from skin cancer data
were one of the first estimations based on empirical clinical studies.
Identifying very low alpha/beta for prostate cancer has attracted
stereotactic hypofractionated radiosurgery as an effective therapeutic
modality. In this review we discuss the pros and cons of the trials and
empirical studies and it looks they are complementary to one another.
Keywords: Randomized clinical trials; Empirical radiotherapy; Head and neck cancer
In oncology, including radiotherapy, there is a
general belief and paradigm that randomized clinical trials (RCTs) have
been emerged as a major or even the only source of evidence based
clinical guidelines. It means that any recommendations of specific
medical procedures should rely on evidence for benefits and costs for
patients. Different RCTs “evidences" have been uncritically for major
changes in the treatment strategy. However, in physics cause-and-effect
relationship is clear, whereas in oncology and radiotherapy is not
simple and easy to be established. Genetic and biological nature of
malignant tumors and patients who suffer from them is widely individual
and complicated, and a final common pathway, if recognized, is initiated
from wide range of possible triggers [1].
For over last 50 years’ experience with RCTs in
radiation oncology many uncertainties and doubts have been a rised, and
important question, which expected to be is do they indeed play a major
or rather modest role in the progress in clinical radiotherapy, and
should they be continued based on classic protocols [2]. This topic was one of important discussion during conference of American Radium Society in May 2017.
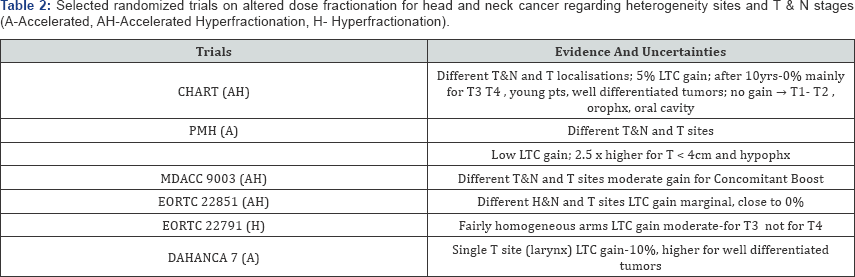
Well-known RCTs of head and neck tumors are reviewed
to consider whether have they had an important impact on therapeutic
gain in radiation oncology. Identifying hypoxic cells in human tumors
various approaches were investigated to eliminate hypoxic cell
subpopulation. Since middle 70-ties to the middle of 80-ties to increase
tumor oxygen delivery, and to strengthen efficacy of conventional dose
fractionation, Hyperbaric Oxygen Therapy (HBO) was tested in 19 clinical
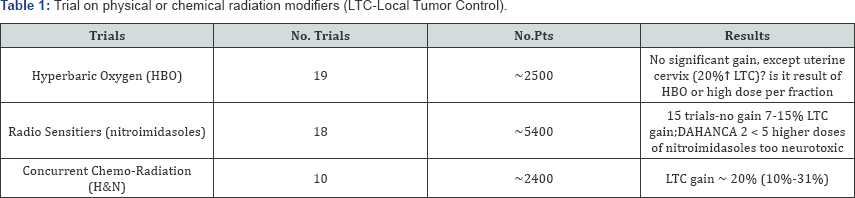
trials (Table 1) which included all together about 2400 patients with various tumor sites and stages [3,4].
The early studies failed to show pronounced improvement in outcome,
later studies gave conflicting results, with either showing some benefit
or no significant gain. Cervix cancer was the only exception and the
MRC trial showed 20% gain in the local tumor control in the HBO arm.
However, it has been difficult to conclude whether this gain resulted
from the HBO or due to the use of high fraction doses ( Table 1).
Because it was no longer easy to recruit a high number of patients to
such trials, they were discontinued in favor of agents which
specifically target the hypoxic cells and sensitize these cells to
radiation. In vitro studies demonstrated that highly electron-affinic
nitroaromatic compounds can preferentially radiosensitize hypoxic cells.
Different compounds were tested, i.g. misomidasol, pimonidasol,
sanazole and others. In this promising field, 18 trials which recruited
about 5000 patients were designed and carried out. Once again, in the 15
trials no therapeutic gain was noted, except DAHANCA 5 trial in which
nimorazole in head and neck cancer was used it resulted in 16%
improvement in the LTC [4].
Failure to note any benefit was generally caused by the fact that the
drug doses were found too low, and a higher doses necessary for
effective radiosensitization produced high risk of severe neurotoxicity.
Once again, large series of these RCTs were more or less disappointing.
When overall treatment time (OTT) in radiotherapy for
head and neck cancerswas recognized and well documented as one of a
majordeterminant of therapeutic benefit [5-7],
various altered fractionation schedules were designed as a challenge to
conventional fractionation with relatively long OTTs. More than 33
RCTs, recruited over 11000 head and neck cancer patients, have been
carried out for over 25 years. Putting it in a mildly way, expectation
that one or some of altered schedules may become as a "Holy Grail” for
head and neck cancers of various sites and stages seems to be a bit
naive. If fact, some of the RCTs showed even inverse results [8,9].
CHART trial has shown average LTC gain of about 5% (which fell down to
0% after 10-years of follow- up), and it was noted mainly for T3-T4
tumors, young patients (< 50 years) with well differentiated cancer,
but not for T1-T2 cancers of oropharynx, oral cavity and hypopharynx.
However, similar efficacy 54Gy in 12 days and 66Gy in 45 days, may
suggest that "the shorter is as effective as the longer”. Using L-Q
model (α/β value = 10Gy) the CHART NTD dose is 51.7i 20 if
given in 2.0Gy fractions. Total physical dose of 66Gy in control arm,
corrected for accelerated repopulation (0.6Gy/day) above week 3 of
irradiation [7], decreases to biologically equivalent dose (NTD) of 51.6izoGy20.
Therefore, biological equivalent doses were almost the same in both
arms, and mature results of this trial surprisingly revealed a few
percents higher gain in favor of the control arm [10].
On the contrary in the PMH trial 2.5 times higher LTC gain was noted
for small tumors (< 4cm), and mainly for the hypopharynx (Table 2).
Among many RCTs, the DAHANCA-7 trial is one of the most reliable since
it included laryngeal cancersonly, and it was carried out only in the
Danish cancer centers using the same protocol [11].
Due to shortening OTT by one week average 10% gain in the LTC was
noted, but, for the select subgroup of well differentiated tumors the
LTC increased to about 20%.
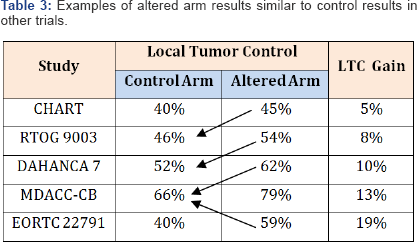
Evaluation of the RCTs results may lead to a bit confusing comparison (Table 3).
In some trials the LTC rates in altered arm are close to the LTC in the
control arm of other trials. Results of two meta analyses (MARCH 1 and
2) have clearly shown that previously awaited "Holy Grail” do not
show-up [8,9].
Twenty one of the 50 trials (42%) were potentially eligible, but
finally, only 15 trials (30%) with 6515 patients were included to the
meta analysis MARCH, and absolute 5-year LTC benefit was 3-4% (8% for
hyperfractionation but only 2% for accelerated schedules). It is
difficult to understand why only one/third of the trials was selected to
the meta analyses. It suggests that excluded trials did not fulfil
methodological requirements. It looks like more than 60% of the trials
did not provide any contribution to progress.
Important step forward in radiotherapy has been
expected due to studies on molecular tumor profiles as predictors and
prognostic factors. From Buffa et al. [12]
analysis fairly homogenous CHART patient' clusters shown that
configuration of negative p53, Bcl2-, with low Ki-67 and low CD31
predicts the LTC gain significantly higher than 5%. It may suggest that
therapeutic gain expected in many altered trials is hidden, and in fact
it may exist not for all different H&N tumors but for clinically and
biologically selected homogeneous group of patients.
Combined chemotherapy with radiation has raised a
flurry of interest as attractive therapeutic modality regarding an
improvement of both LTC and disease-free survival (DFS). Once again,
meta analyses (MACH-NC, MACH-CH) showed [13,14]
lower than modest average therapeutic gain of only 4%, (2% improvement
for neoadjuvant and adjuvant chemotherapy, and promising benefit of 12%
for concurrent chemoradiation). Denis et al. [15]
study on efficacy of chemoradiotherapy for advanced oropharyngeal
cancer has shown 23% increase in the 5-year LTC. This is a good example
that when the RCT concentrates on single tumor type and site,
therapeutic benefit can raise significantly. In contrary, when the RCTs
include various tumor sites and stages which are the source of wide
range of the initial number of cancer stem cells, then a real LTC gain
can be washed away.
In a few trials misleading negative results have been
reported. For example PORT meta analysis has shown no therapeutic
benefit for postoperative radiotherapy for lung cancer patients. Thames
(personal communication) reanalyzing the results of this meta analysis
pointed out that a weak point was to mix together the 2D - 60Co and 3D-conformal results. When his cut-off the 60Co
results and focused on the 3D trials only then reported negative
results turned into significant 10% long-term therapeutic LTC gain.
Another example of the misleading results is the
p-CAIR trial focused on accelerated (7-days-a week) versus conventional
(5-days-a-week) postoperative radiotherapy for H&N patients with
high risk of local recurrence carried out by Suwinski et al. [16].
At the first glance, the authors noted no significant LRC difference
between two arms of the trials. However, they designed own numerical
molecular scoring system, i.e. high EGFR=1, low p53=1, low Ki-67=1, and
low nm-23=1. For patients with total score higher than 2, 40% increase
in the LRC was found in favor of accelerated (7d/wk) schedule. Better
outcome of HPV positive patients was well documented at least for
oropharyngeal cancer, but this factor was not accounted for any previous
trials.
Generally, results of various fractionation schedules
used in the trials do not allow to distinguish the effect of dose from
that of the OTT. Survey of clinical papers in two journals (Radiother
Oncol and J Radiat Oncol Biol Phys) has shown that only one-third of
them contain a satisfactory description of how the dose was specified [17].
Although, authors generally agree that the RCTs in radiotherapy improve
its efficacy, systemic interpretation of the results published in the
literature is quite difficult and it should be taken with some grains of
salt. Among 141 RCTs on therapy of advanced breast cancer (about 26,300
patients), reported on ASCO between 1984-1993, only three (2.1%) showed
significant benefit from the experimental treatment arm. It leads the
readers to the impression that specific results recommended as evidence
based, are in fact general but not specific guidelines, and how to treat
individual patients is still considered as a statistically average
persons.
There is no doubt that the LTC gain with increasing
total dose is almost axiomatic for radiotherapy, but variations in
natural tumor biology may often dominate over the effect of dose
fractionation. In biostatistics of the RCTs the sample size is not only
important, but also its nature also [1,2,17,18].
Methodological problems sometimes make conclusions uncertain and for
that reason they are likely excluded from the meta analyses, (i.g.
MARCH).
Factor as tumors biology, cell density,
radiosensitivity, not constant rate of cell killing after daily
fractions are highly heterogeneous. But, even if the RCTs are well
designed and analyzed, the results could be somewhat puzzling since
within a specific tumor stage TCP may differ significantly.
One of the Achilles heel of the RCTs seems to be the
belief that relatively wide range of tumor sites and stages enrolled to
each arm of the trial will alike respond to irradiation schedule. It's
difficult to understand why TNM tumor stage still remains as axiomatic
criterion of inclusion to the trial, whereas initial number of tumor
stem cells (indirectly represented by tumor volume) is in fact radiation
targets. Therefore it seems inconsistent to tailor dose fractionation
to the T or N stage. This indisputable fact is however ignored. Although
at the first glance, randomized arms may look homogeneous clinically
but there is about 10-fold difference in the initial number of tumor
stem cells between the smallest and the largest tumors, within T2 stage,
and much larger for higher T stages but a total dose given to all cases
within each arm is the same. If, just by chance, in the control arm
will be allocated more smaller tumors in each T category, and on the
contrary, more larger tumors will be in the altered arm, it may likely
lead to higher efficacy (higher LTC) in the control than in the altered
one. Should such result be therefore considered as "an evidence” or
rather as "an illusion”? Therefore it sounds logical that dose
fractionation should be tailored to the volumetric staging which
reflects number of the stem cells, but no longer to the T and N stages.
Usually the results of the trials are presented as an
average and usually actuarial but not crude end-points in the selected
time of the follow-up. Sometimes one can lose valuable information’s
that are spread around and beyond such point. Glatstein [1] called it as a “Tyranny of the median".
Major problem with the median value is that the rest of survival curve
is usually ignored. Furthermore, the probability for outcome is related
to whole group but not for individuals. Nevertheless, Bentzen wittily
and also rightly pointed out that “Evidence of lack of significance, does not necessarily means the lack of evidence" On the other hand, statistical significance not always corresponds with clinical importance.
Considering at least major doubts and uncertainties
of the RCTs, there is not unanimous answer to the question whether
randomized clinical trials should not be considered as evidence based
milestones of progress in radiotherapy, although it may seem that they
have played a modest role. Glatstein [1] has used elegant Latin proverb “Caveat Emptor",
which means a kind of warning that one should be cautious to make
unequivocal conclusions. Although evidence based guidelines are
addressed to clinicians, evidence has to be measured and weighed
carefully, and frequently, and it requires clinical experience, common
sense and logic. It does not, however, means that evidence should be
ignored and dismissed out from practical radiotherapy.
For about 10 decades empiricism has been a source of
knowledge, growing experience and also progress in radiotherapy, but not
as an alternative to RCTs. A few decades ago, Fowler mentioned that - “If
radiotherapist had to await for fully scientific evidence basis for
treating, for first patient radiotherapy would not have started yet".
According to David Hume (XVIII century) “empircism means the best
contact between one’s understanding of knowledge and the world and it is
not the point at which a mathematical proof crystalizes". In radiation
oncology empiricism comes from generally accumulated clinical experience
based, on “what has worked in the pastand what has not". Strong
and important attribute of the empiricism are retrospective clinical
studies, which for decades have been a source for growing clinical
experience to select tumor volumes, dose and fractionation, timing,
treatment techniques to design standards, long before the RCTs have
developed.
In XX century, Gray Laboratory was a “Mecca"
of experimental and clinical radiobiology. Almost all fundamental
radiobiological mechanisms of tumor and normal tissue response to
radiation were recognized, quantified as a basic rationale for clinical
radiotherapy. In late 60-ties Fletcher proposed modern clinical
radiotherapy based on radiobiology principles which became a major
milestone in radiotherapy. For example, utilizing own clinical
observations, Fletcher pointed out that if dose per week is not higher
than 10Gy, then in about 60% of H&N cancer patients healing of the
acute mucosal reaction (confluent mucositis) already occurs at the end
of week 6 of conventional irradiation. His observations were confirmed
by the results of many studies. In his time, accelerated repopulation
was not discovered yet, but he intuitively and indirectly suggested that
it plays important role in response of normal epithelium to
fractionation, and the response is so intensive that effect of daily
fraction of 2Gy can be neutralized by this process.
During 60-ties and 70-ties two cancer centers in
Poland gathered over 1000 skin cancer patients treated with one of the
seven different fractionation schedules from a single dose of 18-25Gy to
70Gy in 47 fractions [5,19].
In none of the RCTs such wide range of doses has been used yet. In
fact, it is methodolically not possible. This large set of
non-randomized empirical retrospective data [5,6,19,20], were later effectively used to:
a) Question reliability of the Strandquist formula
for dose-time relationship and it showed that the exponent for time (T)
in his formula should be higher than 0.33. It was the first indirect
sign that process of accelerated repopulation exist and it should not be
ignored;
b) Demonstrate that tumor volume as a one of the major determinant of effective dose fractionation (Figure 1); (small tumors can be eradicated even by a single dose whereas larger needs a number of fraction doses);

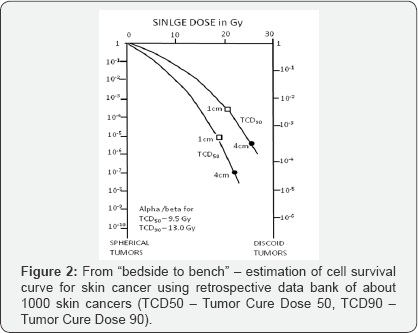
c) To estimate cell survival curves fromclinical data as a bridge between bench and bed site (Figure 2);
d) Estimate alpha/beta values for skin cancer (α/β = 13.0Gy) and skin necrosis
(α/β = 7.4Gy), which were one of the first published in the literature (Figure 3);
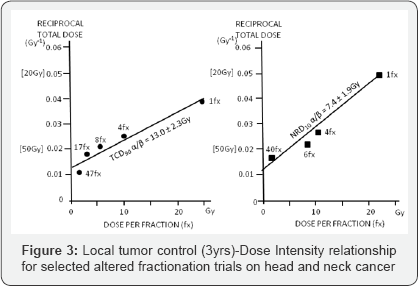
e) Prove that time factor is much more important for
treatment outcome than it was previously assumed and that exponent for
time depends on OTT itself and in the Strandquist' and Ellis' formulas
this factor was underestimated.
All these findings came from a single retrospective clinical data set.
Quantitative analyses of a large retrospective series
of head and neck cancer irradiated in Gliwice have shown that overall
treatment time (OTT) is one of the major determinant of treatment
outcome. Cell kill effect caused by about 0.6Gy daily fraction of 2.0Gy
is neutralized by accelerated repopulation of the survived clonogenic
cells [6,7]. Shortening the OTT results in an increase in the LTC, what has led to a milestone practical guideline that: "It
is more effective to begin radiotherapy on Monday or Tuesday, and worse
(never do it) to complete therapy on Monday or Tuesday” - (it means the last 2-3 fractions should be used as a second daily fraction in the previous week).
Although RCTs on altered fractionation failed to show
pronounced therapeutic benefit, they were a source of important
radiobiological information that accelerated repopulation previously
estimated as a constant Drep = 0.6Gy/day, in fact is increasing during
irradiation and it depends on time OTT itself, with the increase to even
1.4-1.6Gy/days at the end of the week 6 of irradiation. It means that
10Gy at the first week of irradiation is not biologically equivalent to
10Gy at the week 6, because in the week 6 cell kill effect of at least
3.5-4Gy (2.5 days x 1.4Gy) of 10Gy/week is balanced by repopulation
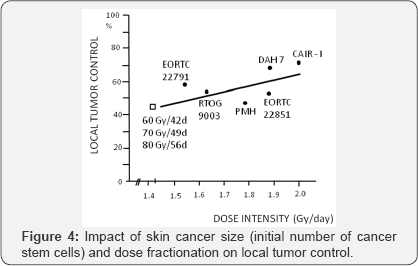
during weekend and thereafter [7]. The RCTs results have shown that attention should be focused on Dose Intensity
(DI) (number of Gray per unit of time, e.g. number of Gy/day), which is
more reliable parameter corresponding with treatment efficacy then Dose
Escalation (DE). The latter one simply expresses an increase in
physical dose. Although, 60Gy in 42 days, 70Gy in 49 days and 80Gy in 56
days illustrate dose escalation from 60Gy to 80Gy, but the same DI of
these three schedules (DI = 1.43Gy/d) means that they are biologically
equivalent. Therefore, the DI seems more handy than DE to evaluate
biological efficacy of different fractionation schedules (Figure 4).
All these, practically useful information's came from empirical, often
retrospective studies and have been used as rationale to design altered
fractionation RCTs carried out through more than two decades.
A few years ago, Fowler, has mentioned that radiotherapy is like a "Round Game",
that means, some rules and methods abandoned in the past, nowadays are
coming back to the market. Fowler together with Ritter & Bentzen [21-23] using retrospective data have estimated unexpectedly low alpha/beta value of less than 2.0Gy for prostate cancer.
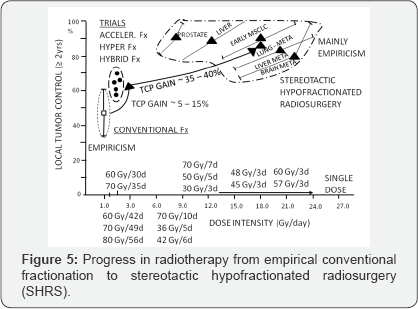
Revival a single dose or few large fractions as a high-tech stereotactic radiotherapy has been a real milestone "back to future"
in radiotherapy. Nowadays, large single dose of 8-29Gy or a few
fractions of 7 up to even 20Gy are more and more widely used as radical
therapy of primary or metastatic brain tumors, head and neck, lung,
pancreas, liver or prostate cancer. Results look spectacular and provide
high rate of 80-95% of at least 2 year LTC. This is undoubtedly an
important milestone in
radiotherapy, with its empirical roots. Figure 5 illustrates this enormous “gain-jump"
in curative radiation oncology, although a majority studies have a long
empirical history and those on stereotactic hypofractionated
radiotherapy also remains mainly empirical and RCTs just have begun.
Despite many years of empirical and randomized studies, somewhat epical
question - “Mirror, mirror at the wall-tell which therapy is the best at
all" -still remains unanswered.
During many decades of radiotherapy we have learned that “cause-and-effect relationships"
are not simple. Malignant tumors and humans biology is widely
individual, and recognizable final common pathway is initiated from wide
range of possible triggers [1].
Nobody can settle that whether empirical radiotherapy is advantageous
or inferior alternative to the RCTs, because in fact they are
complementary to one another. Empirical retrospective studies should not
be ignored as a source of practical importance although they are not
randomized.
The RCTs results, even significant, are not always
useful to design individually personalized radiotherapy alone or as a
part of systemic therapy. It should be remembered that about 80% of
patients stay out of any RCTs. There is plausible expectation that
genomics, proteomics and molecular tumor profiles shell influence
philosophy and tailoring radiation oncology. Despite of preliminarily
unsatisfied results of the CHART or p-CAIR show that molecular profiles
allowed to select well defined clusters of patients with the LTC much
higher than average median. Already translation research strongly
influences present and prospective progress in radiotherapy. There is no
doubt the RCTs should be continued to check and prove empirical
findings but its methodological rules and criteria need likely to be
updated. It seems that so-called “Feedback Trial" might be a reasonable
solution. It means, that patients with molecular profiles estimated
prior to therapy should be enrolled into the trial and randomized based
on volumetric, but not TNM staging. Longterm results categorized as
winners (cured) or losers (failures) should be confronted back with
molecular profiles within each category and cross-checked for winners
and losers to define specific molecular and/or genetic markers being a
strong specific predictors for each end-point. It likely seems the only
rational way to move from averages to evidence bases, individually
personalized effective radiation therapy.
To know more about Open Access Journal of
Head Neck & Spine Surgery please click on:
To know more about Open access Journals
Publishers please click on : Juniper Publishers
Comments
Post a Comment